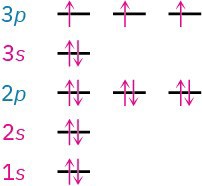1.3 Atomic Structure: Electron Configurations
The lowest-energy arrangement, or ground-state electron configuration, of an atom is a list of the orbitals occupied by its electrons. We can predict this arrangement by following three rules.
RULE 1
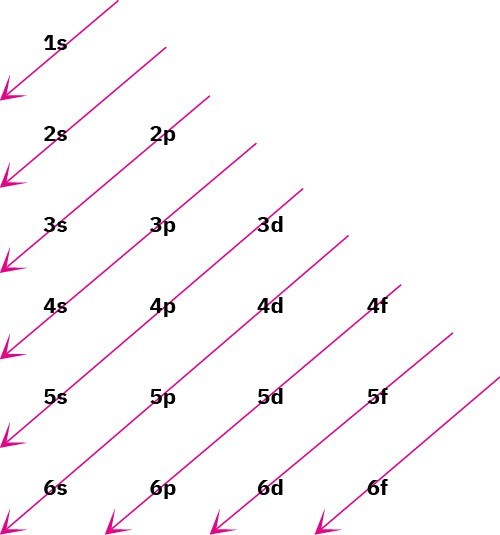
The lowest-energy orbitals fill up first, 1𝑠 → 2𝑠 → 2𝑝 → 3𝑠 → 3𝑝 → 4𝑠 → 3𝑑, according to the following graphic, a statement called the Aufbau principle. Note that the 4s orbital lies between the 3p and 3d orbitals in energy.
RULE 2
Electrons act in some ways as if they were spinning around an axis, somewhat as the earth spins. This spin can have two orientations, denoted as up (↑) and down (↓). Only two electrons can occupy an orbital, and they must have opposite spins, a statement called the Pauli exclusion principle.
RULE 3
If two or more empty orbitals of equal energy are available, one electron occupies each with spins parallel until all orbitals are half-full, a statement called Hund’s rule.
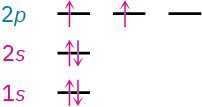
Some examples of how these rules apply are shown in Table 1.1. Hydrogen, for instance, has only one electron, which must occupy the lowest-energy orbital. Thus, hydrogen has a 1s ground-state configuration. Carbon has six electrons and the ground-state configuration 1s22s22px12py1, and so forth. Note that a superscript is used to represent the number of electrons in a particular orbital.
Table 1.1 Ground-State Electron Configurations of Some Elements
|
Hydrogen |
1 |
|
|
Carbon |
6 |
|
|
Phosphorus |
15 |
|
Problem 1-1
What is the ground-state electron configuration of each of the following elements: (a)
Oxygen (b) Nitrogen (c) Sulfur
Problem 1-2
How many electrons does each of the following biological trace elements have in its outermost electron shell?
(a) Magnesium (b)
Cobalt (c) Selenium