14.5 Characteristics of the Diels–Alder Reaction
The Dienophile
The Diels–Alder cycloaddition reaction occurs most rapidly if the alkene component, called the dienophile (“diene lover”), has an electron-withdrawing substituent group. Thus, ethylene itself reacts sluggishly, but propenal, ethyl propenoate, maleic anhydride, benzoquinone, propenenitrile, and similar compounds are highly reactive. Note also that alkynes, such as methyl propynoate, can act as Diels–Alder dienophiles.
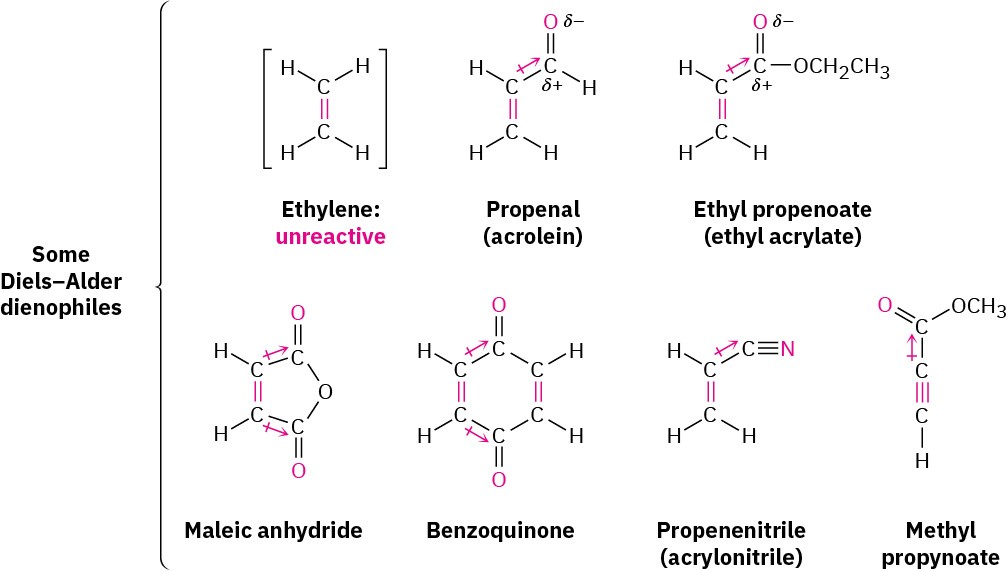
In all cases, the double or triple bond of the dienophile is adjacent to the positively polarized carbon of an electron-withdrawing substituent. As a result, the double-bond carbons in these substances are substantially less electron-rich than the carbons in ethylene, as indicated by the electrostatic potential maps in Figure 14.9.
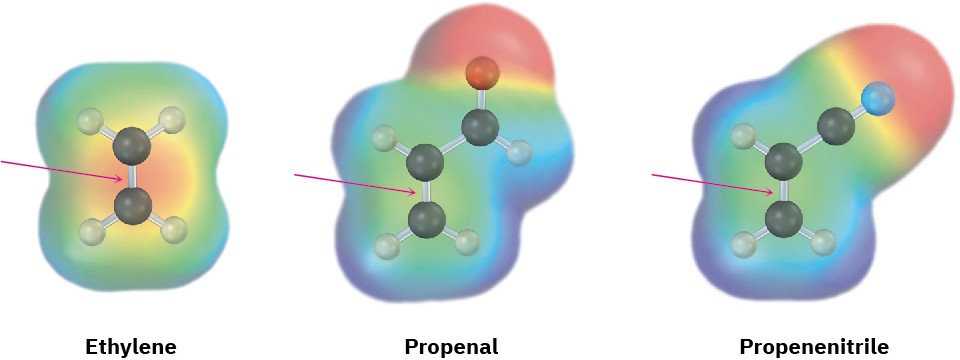
Figure 14.9Electrostatic potential maps of ethylene, propenal, and propenenitrile show that electron-withdrawing groups make the double-bond carbons less electron-rich.
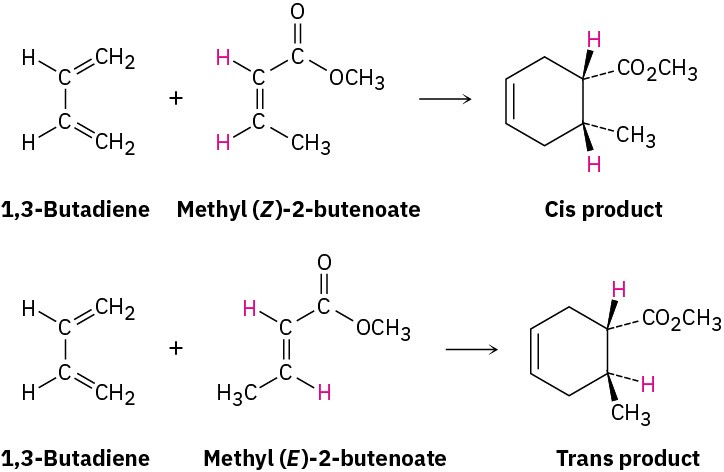
One of the most useful features of the Diels–Alder reaction is that it is stereospecific, meaning that a single product stereoisomer is formed. Furthermore, the stereochemistry of the dienophile is retained. If we carry out cycloaddition with methyl cis-2-butenoate, only the cis-substituted cyclohexene product is formed. With methyl trans-2-butenoate, only the trans-substituted cyclohexene product is formed.
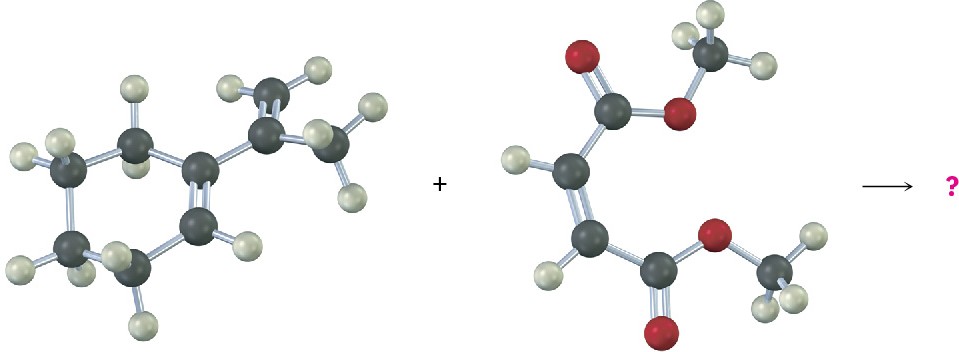
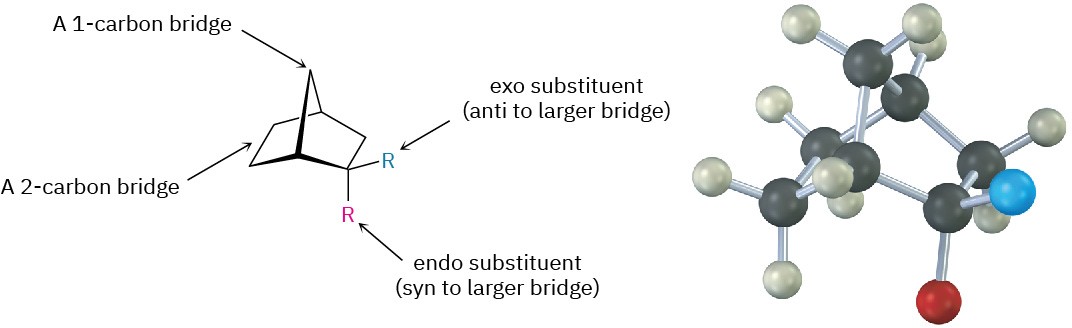
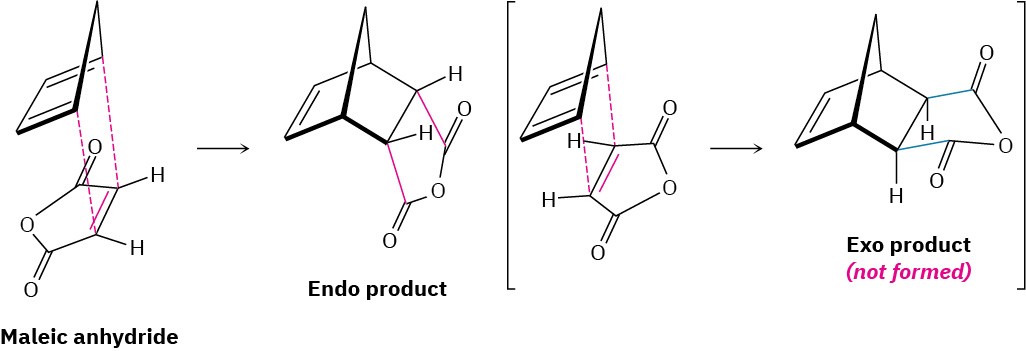
Another stereochemical feature of the Diels–Alder reaction is that the diene and dienophile partners orient so that the endo product, rather than the alternative exo product, is formed. The words endo and exo are used to indicate relative stereochemistry when referring to bicyclic structures like substituted norbornanes (Section 4.9). A substituent on one bridge is said to be endo if it is syn (cis) to the larger of the other two bridges and is said to be exo if it is anti (trans) to the larger of the other two.
Endo products result from Diels–Alder reactions because the amount of orbital overlap between diene and dienophile is greater when the reactants lie directly on top of one another, so that the electron-withdrawing substituent on the dienophile is underneath the diene double bonds. In the reaction of 1,3-cyclopentadiene with maleic anhydride, for instance, the following result is obtained:
Worked Example 14.2Predicting the Product of a Diels–Alder ReactionPredict the product of the following Diels–Alder reaction:
 StrategyDraw the diene so that the ends of its two double bonds are near the dienophile double bond. Then form two single bonds between the partners, convert the three double bonds into single bonds, and convert the former single bond of the diene into a double bond.Because the dienophile double bond is cis to begin with, the two attached hydrogens must remain cis in the product.Solution
StrategyDraw the diene so that the ends of its two double bonds are near the dienophile double bond. Then form two single bonds between the partners, convert the three double bonds into single bonds, and convert the former single bond of the diene into a double bond.Because the dienophile double bond is cis to begin with, the two attached hydrogens must remain cis in the product.Solution
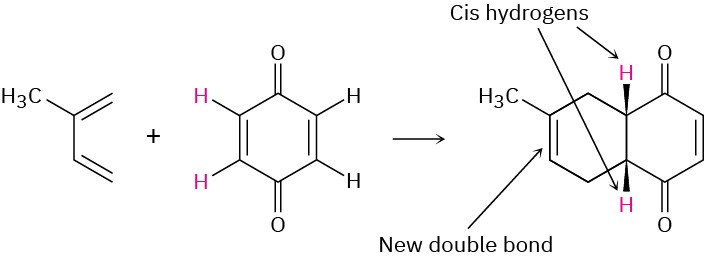
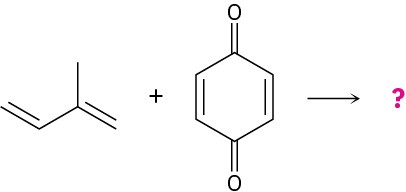
Problem 14-7
Predict the product of the following Diels–Alder reaction:
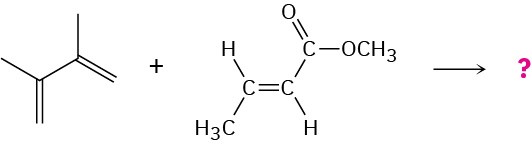
The Diene
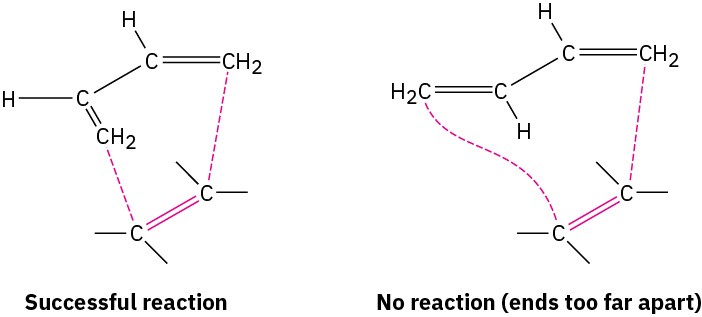
Just as the dienophile component has certain constraints that affect its reactivity, so too does the conjugated diene component. The diene must adopt what is called an s-cis conformation, meaning “cis-like” about the single bond, to undergo a Diels–Alder reaction. Only in the s-cis conformation are carbons 1 and 4 of the diene close enough to react through a cyclic transition state.
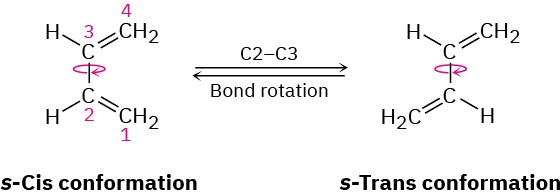
In the alternative s-trans conformation, the ends of the diene partner are too far apart to overlap with the dienophile p orbitals.
Two examples of dienes that can’t adopt an s-cis conformation, and thus don’t undergo Diels–Alder reactions, are shown in Figure 14.10. In the bicyclic diene, the double bonds are rigidly fixed in an s-trans arrangement by geometric constraints of the rings. In (2Z,4Z)- 2,4-hexadiene, steric strain between the two methyl groups prevents the molecule from adopting s-cis geometry.
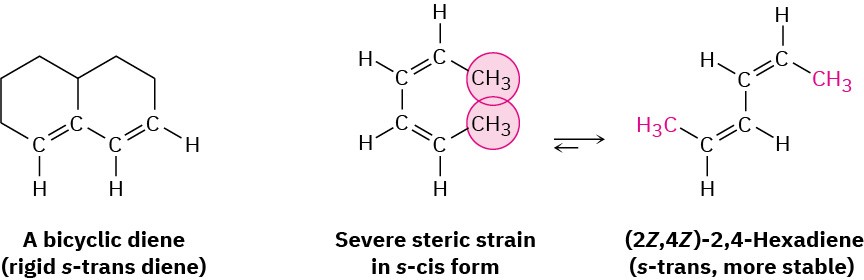
Figure 14.10Two dienes that can’t achieve an s-cis conformation and thus can’t undergo Diels–Alder reactions.
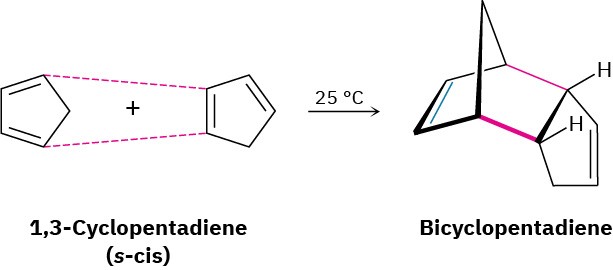
In contrast to these unreactive dienes that can’t achieve an s-cis conformation, other dienes are fixed only in the correct s-cis geometry and are therefore highly reactive in Diels–Alder cycloaddition. 1,3-Cyclopentadiene, for example, is so reactive that it reacts with itself. At
room temperature, 1,3-cyclopentadiene dimerizes. One molecule acts as diene and a second molecule acts as dienophile in a self-Diels–Alder reaction.
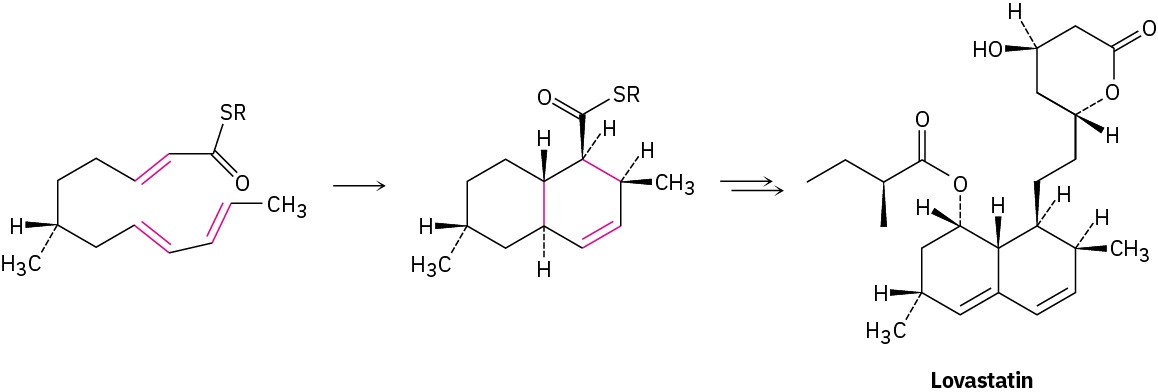
Biological Diels–Alder reactions are also known but are uncommon. One example occurs in the biosynthesis of the cholesterol-lowering drug lovastatin (trade name Mevacor) isolated from the bacterium Aspergillus terreus. The key step is the intramolecular Diels–Alder reaction of a triene, in which the diene and dienophile components are within the same molecule.
Problem 14-8
Which of the following alkenes would you expect to be good Diels–Alder dienophiles?
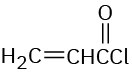
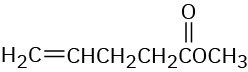
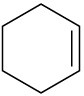 (a)(b)(c)(d)
(a)(b)(c)(d) 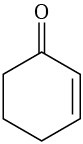 (e)
(e) 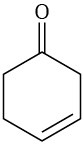
Problem 14-9
Which of the following dienes have an s-cis conformation, and which have an s-trans conformation? Of the s-trans dienes, which can readily rotate to s-cis?
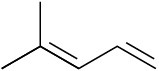 (a)
(a) 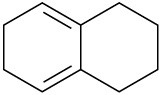 (b)
(b) 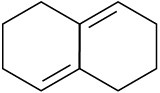 (c)
(c)
Problem 14-10
Predict the product of the following Diels–Alder reaction: