7.11 Evidence for the Mechanism of Electrophilic Additions: Carbocation Rearrangements
How do we know that the carbocation mechanism for electrophilic addition reactions of alkenes is correct? The answer is that we don’t know it’s correct; at least we don’t know with complete certainty. Although an incorrect reaction mechanism can be disproved by demonstrating that it doesn’t account for observed data, a correct reaction mechanism can never be entirely proven. The best we can do is to show that a proposed mechanism is consistent with all known facts. If enough facts are accounted for, the mechanism is probably correct.
One of the best pieces of evidence supporting the carbocation mechanism for the electrophilic addition reaction was discovered during the 1930s by F. C. Whitmore of Pennsylvania State University, who found that structural rearrangements often occur during the reaction of HX with an alkene. For example, reaction of HCl with 3-methyl-1- butene yields a substantial amount of 2-chloro-2-methylbutane in addition to the “expected” product, 2-chloro-3-methylbutane.
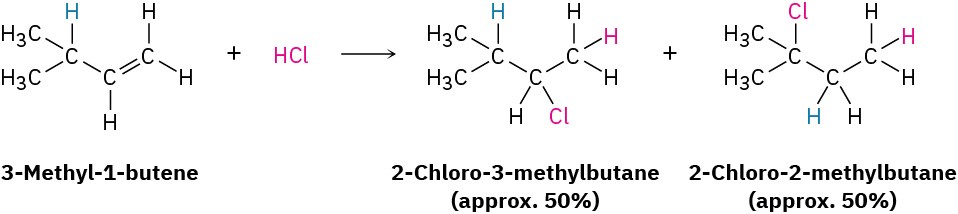
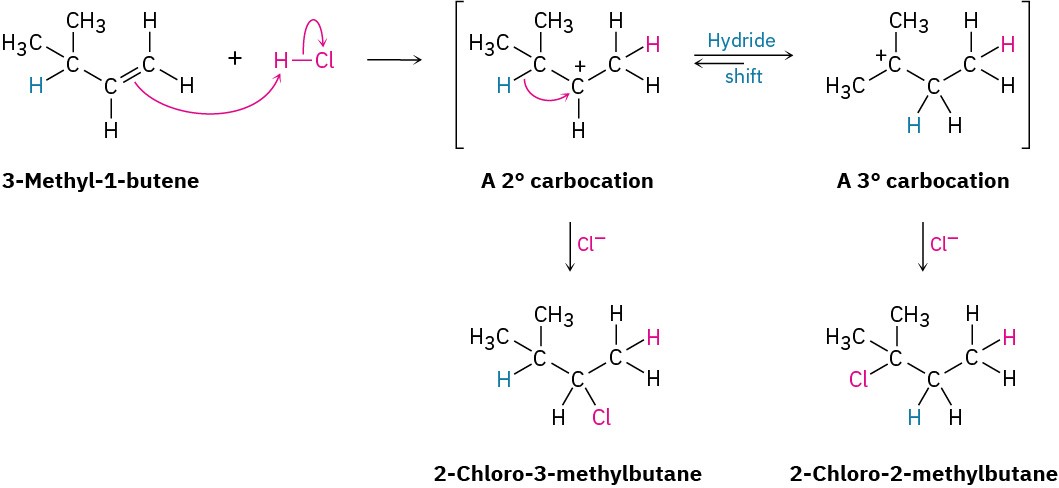
If the reaction takes place in a single step, it would be difficult to account for rearrangement, but if the reaction takes place in several steps, rearrangement is more easily explained. Whitmore suggested that it is a carbocation intermediate that undergoes rearrangement. The secondary carbocation intermediate formed by protonation of 3- methyl-1-butene rearranges to a more stable tertiary carbocation by a hydride shift—the shift of a hydrogen atom and its electron pair (a hydride ion, :H−) between neighboring carbons.
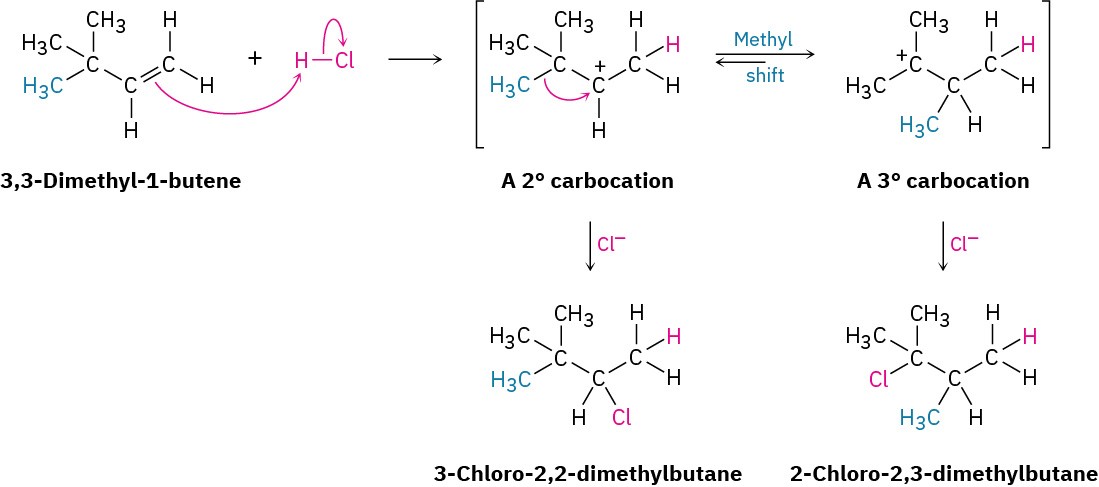
Carbocation rearrangements can also occur by the shift of an alkyl group with its electron pair. For example, reaction of 3,3-dimethyl-1-butene with HCl leads to an equal mixture of unrearranged 3-chloro-2,2-dimethylbutane and rearranged 2-chloro-2,3-dimethylbutane. In this instance, a secondary carbocation rearranges to a more stable tertiary carbocation by the shift of a methyl group.
Note the similarities between the two carbocation rearrangements: in both cases, a group (:H− or :CH3−) moves to an adjacent positively charged carbon, taking its bonding electron pair with it. Also in both cases, a less stable carbocation rearranges to a more stable ion. Rearrangements of this kind are a common feature of carbocation chemistry and are particularly important in the biological pathways by which steroids and related substances are synthesized. An example is the following hydride shift that occurs during the biosynthesis of cholesterol.
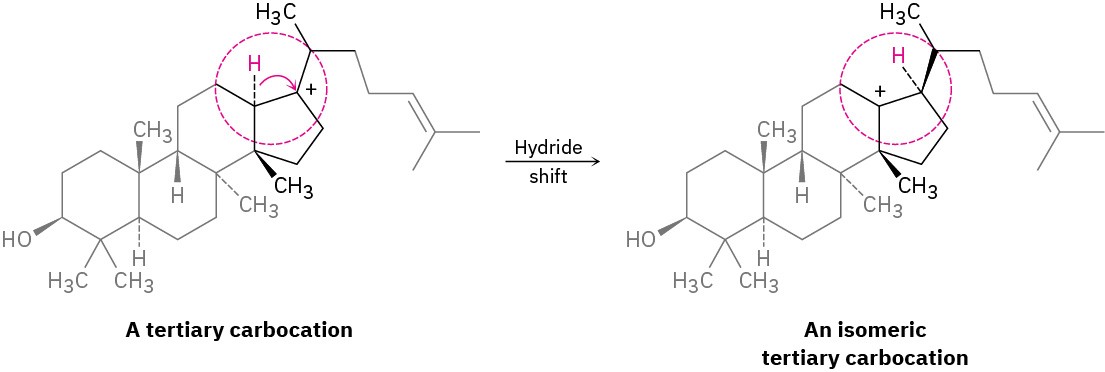
As always, when looking at any complex chemical transformation, whether biochemical or not, focus on the part of the molecule where the change is occurring and don’t worry about the rest. The tertiary carbocation just pictured looks complicated, but all the chemistry is taking place in the small part of the molecule inside the red circle.
Problem 7-21
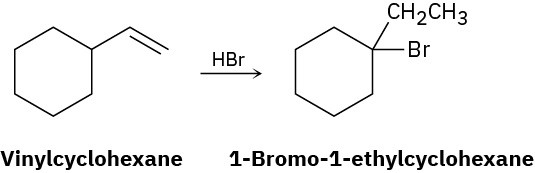
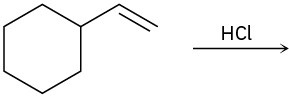
On treatment with HBr, vinylcyclohexane undergoes addition and rearrangement to yield 1-bromo-1-ethylcyclohexane. Using curved arrows, propose a mechanism to account for this result.
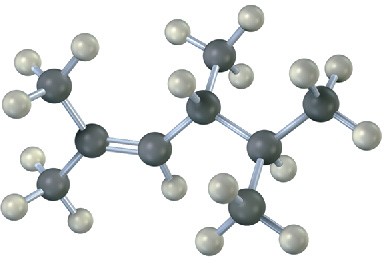
Additional Problems 7 • Additional Problems 7 • Additional Problems Visualizing Chemistry Problem 7-22
Name the following alkenes, and convert each drawing into a skeletal structure: (a)
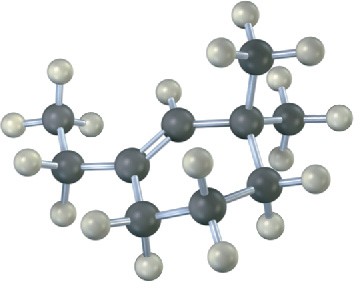
(b)
Problem 7-23
Assign E or Z stereochemistry to the double bonds in each of the following alkenes, and convert each drawing into a skeletal structure (red = O, green = Cl):
(a)
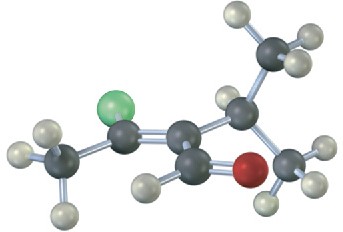
(b)
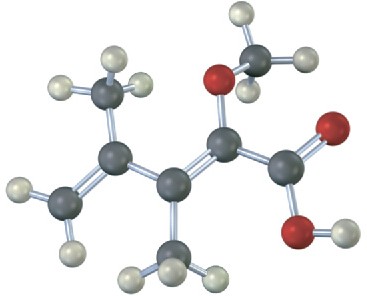
Problem 7-24
The following carbocation is an intermediate in the electrophilic addition reaction of HCl with two different alkenes. Identify both, and tell which C−H bonds in the carbocation are aligned for hyperconjugation with the vacant p orbital on the positively charged carbon.
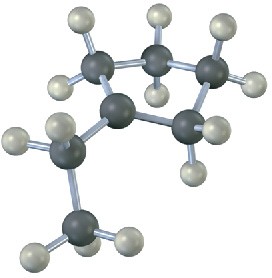
Problem 7-25
The following alkyl bromide can be made by HBr addition to three different alkenes. Show their structures.
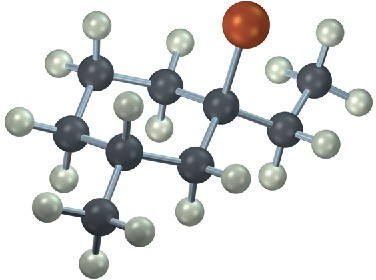
Mechanism Problems
Problem 7-26
Predict the major product and show the complete mechanism for each of the following electrophilic addition reactions.
(a)
(b)
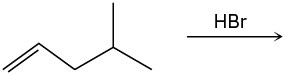
(c)
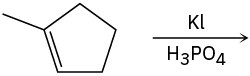
Problem 7-27
Each of the following electrophilic addition reactions involves a carbocation rearrangement. Predict the product and draw the complete mechanism of each using curved arrows.
(a)
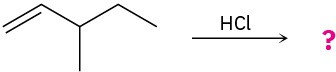
(b)
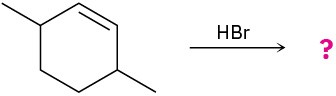
(c)
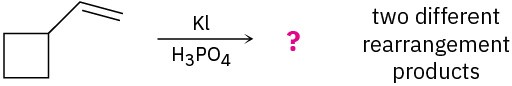
Problem 7-28
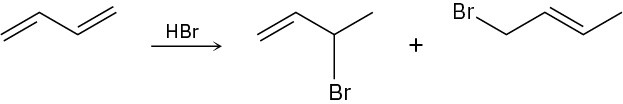
When 1,3-butadiene reacts with 1 mol of HBr, two isolable products result. Propose mechanisms for both.
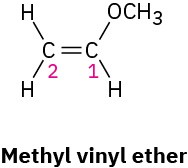
Problem 7-29
When methyl vinyl ether reacts with a strong acid, H+ adds to C2 instead of C1 or the oxygen atom. Explain.
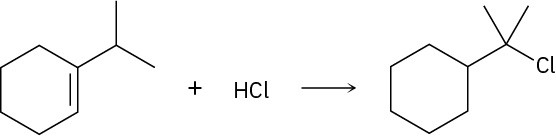
Problem 7-30
Addition of HCl to 1-isopropylcyclohexene yields a rearranged product. Propose a mechanism, showing the structures of the intermediates and using curved arrows to indicate electron flow in each step.
Problem 7-31
Addition of HCl to 1-isopropenyl-1-methylcyclopentane yields 1-chloro-1,2,2- trimethylcyclohexane. Propose a mechanism, showing the structures of the intermediates and using curved arrows to indicate electron flow in each step.
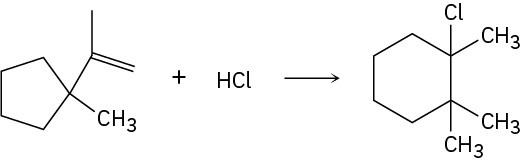
Problem 7-32
Limonene, a fragrant hydrocarbon found in lemons and oranges, is biosynthesized from geranyl diphosphate by the following pathway. Add curved arrows to show the mechanism of each step. Which step involves an alkene electrophilic addition? (The ion OP2O64− is the diphosphate ion, and “Base” is an unspecified base in the enzyme that catalyzes the reaction.)
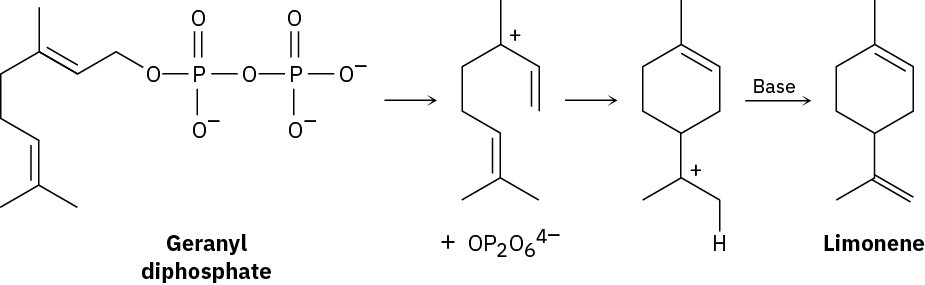
Problem 7-33
epi-Aristolochene, a hydrocarbon found in both pepper and tobacco, is biosynthesized by the following pathway. Add curved arrows to show the mechanism of each step. Which steps involve alkene electrophilic addition(s), and which involve carbocation rearrangement(s)? (The abbreviation H—A stands for an unspecified acid, and “Base” is an unspecified base in the enzyme.)
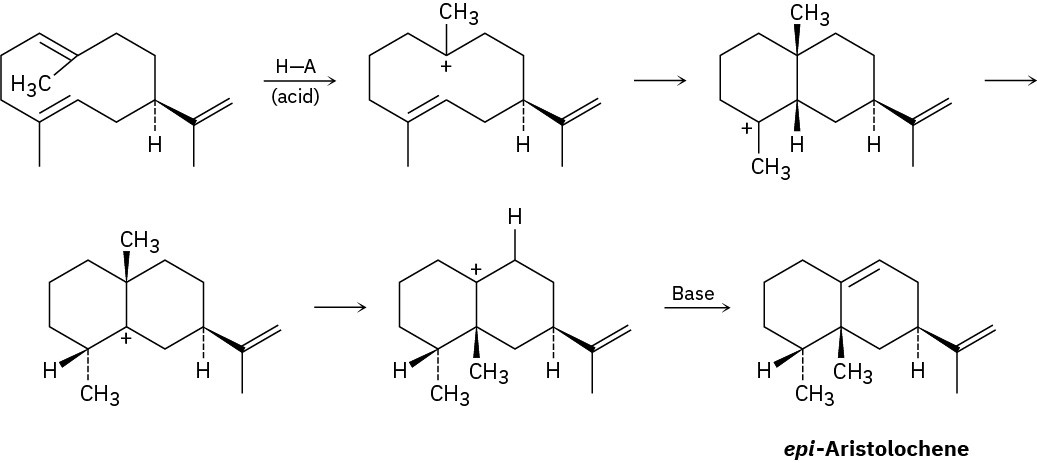
Calculating a Degree of Unsaturation
Problem 7-34
Calculate the degree of unsaturation in the following formulas, and draw five possible structures for each:
(a) C10H16
(b) C8H8O
(c) C7H10Cl2
(d) C10H16O2
(e) C5H9NO2
(f) C8H10ClNO
Problem 7-35
How many hydrogens does each of the following compounds have? (a)
C8H?O2, has two rings and one double bond (b)
C7H?N, has two double bonds (c)
C9H?NO, has one ring and three double bonds Problem 7-36
Loratadine, marketed as an antiallergy medication under the brand name Claritin, has four rings, eight double bonds, and the formula C22H?ClN2O2. How many hydrogens does loratadine have? (Calculate your answer; don’t count hydrogens in the structure.)
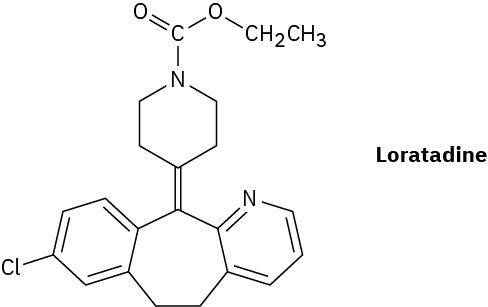
Naming Alkenes
Problem 7-37
Name the following alkenes: (a)
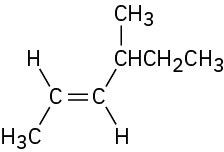
(b)
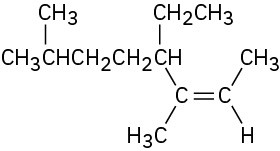
(c)
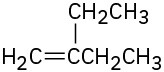
(d)
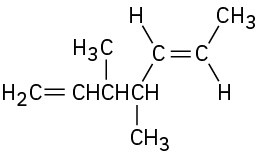
(e)
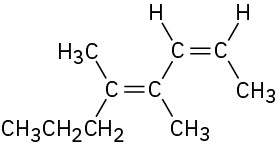
(f)
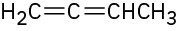
Problem 7-38
Draw structures corresponding to the following systematic names: (a)
(4E)-2,4-Dimethyl-1,4-hexadiene (b)
cis-3,3-Dimethyl-4-propyl-1,5-octadiene (c)
- Methyl-1,2-pentadiene (d)
(3E,5Z)-2,6-Dimethyl-1,3,5,7-octatetraene (e)
3-Butyl-2-heptene (f)
trans-2,2,5,5-Tetramethyl-3-hexene Problem 7-39
Name the following cycloalkenes: (a)
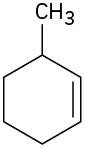
(b)
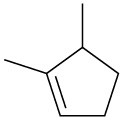
(c)
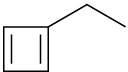
(d)
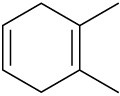
(e)
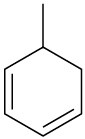
(f)
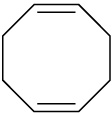
Problem 7-40
Ocimene is a triene found in the essential oils of many plants. What is its IUPAC name, including stereochemistry?
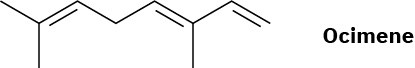
Problem 7-41
α-Farnesene is a constituent of the natural wax found on apples. What is its IUPAC name, including stereochemistry?
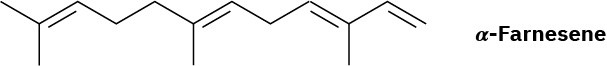
Problem 7-42
Menthene, a hydrocarbon found in mint plants, has the systematic name 1-isopropyl-4- methylcyclohexene. Draw its structure.
Problem 7-43
Draw and name the six alkene isomers, C5H10, including E,Z isomers. Problem 7-44
Draw and name the 17 alkene isomers, C6H12, including E,Z isomers.
Alkene Isomers and Their Stability
Problem 7-45
Rank the following sets of substituents according to the Cahn–Ingold–Prelog sequence rules:
(a)
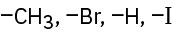
(b)
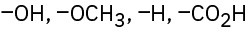
(c)
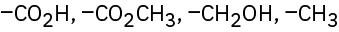
(d)
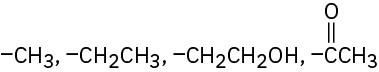
(e)
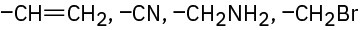
(f)
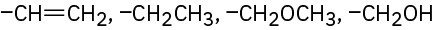
Problem 7-46
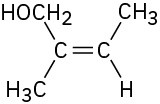 Assign E or Z configuration to each of the following compounds: (a)
Assign E or Z configuration to each of the following compounds: (a)
(b)
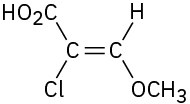
(c)
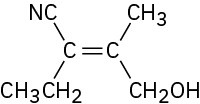
(d)
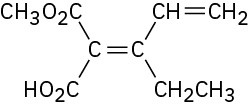
Problem 7-47
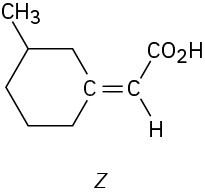 Which of the following E,Z designations are correct, and which are incorrect? (a)
Which of the following E,Z designations are correct, and which are incorrect? (a)
(b)
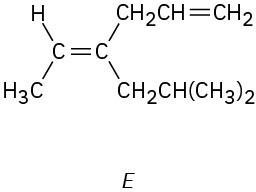
(c)
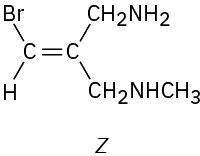
(d)
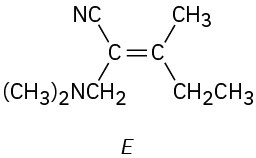
(e)
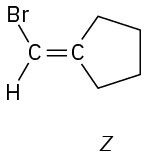
(f)
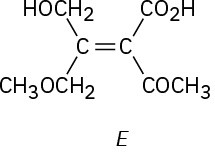
Problem 7-48
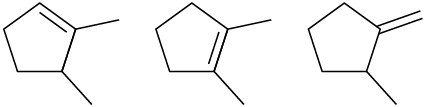
Rank the double bonds according to their increasing stability. (a)
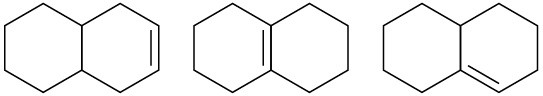
(b)
(c)
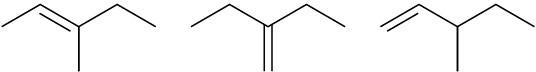
Problem 7-49
trans-2-Butene is more stable than cis-2-butene by only 4 kJ/mol, but trans-2,2,5,5- tetramethyl-3-hexene is more stable than its cis isomer by 39 kJ/mol. Explain.
Problem 7-50
Cyclodecene can exist in both cis and trans forms, but cyclohexene cannot. Explain. Problem 7-51
Normally, a trans alkene is more stable than its cis isomer. trans-Cyclooctene, however, is less stable than cis-cyclooctene by 38.5 kJ/mol. Explain.
Problem 7-52
trans-Cyclooctene is less stable than cis-cyclooctene by 38.5 kJ/mol, but trans-cyclononene is less stable than cis-cyclononene by only 12.2 kJ/mol. Explain.
Problem 7-53
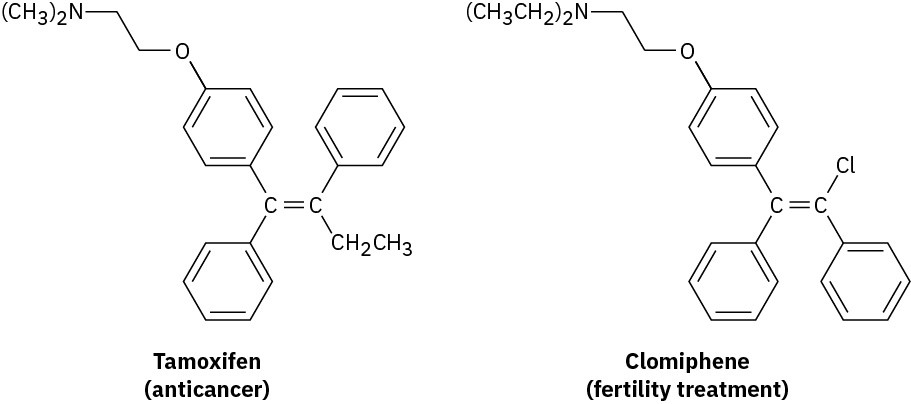
Tamoxifen, a drug used in the treatment of breast cancer, and clomiphene, a drug used in fertility treatment, have similar structures but very different effects. Assign E or Z configuration to the double bonds in both compounds.
Carbocations and Electrophilic Addition Reactions
Problem 7-54
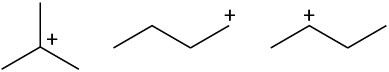
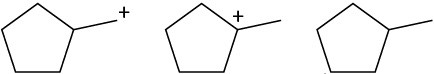
Rank the following carbocations according to their increasing stability. (a)
(b)
(c)
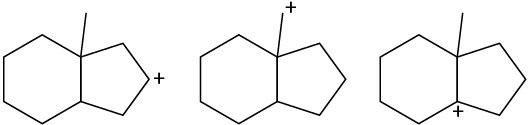
Problem 7-55
Use the Hammond Postulate to determine which alkene in each pair would be expected to form a carbocation faster in an electrophilic addition reaction.
(a)

(b)

(c)

Problem 7-56
The following carbocations can be stabilized by resonance. Draw all the resonance forms that would stabilize each carbocation.
(a)
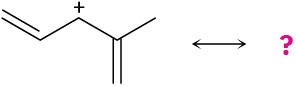
(b)
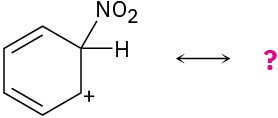
(c)
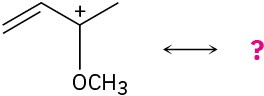
Problem 7-57
Predict the major product in each of the following reactions: (a)
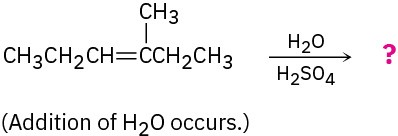
(b)
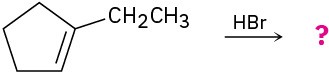
(c)
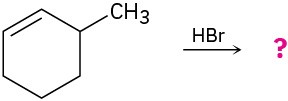
(d)

Problem 7-58
Predict the major product from addition of HBr to each of the following alkenes: (a)
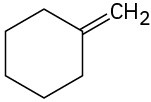
(b)
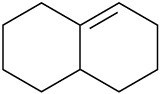
(c)
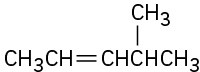
Problem 7-59
Alkenes can be converted into alcohols by acid-catalyzed addition of water. Assuming that Markovnikov’s rule is valid, predict the major alcohol product from each of the following alkenes.
(a)
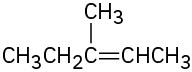
(b)
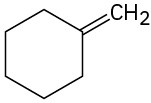
(c)
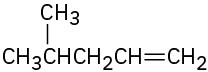
Problem 7-60
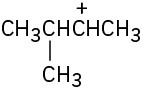
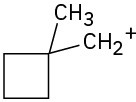
Each of the following carbocations can rearrange to a more stable ion. Propose structures for the likely rearrangement products.
(a)
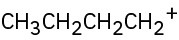
(b)
(c)
General Problems
Problem 7-61
Allene (1,2-propadiene), H2C=C=CH2, has two adjacent double bonds. What kind of hybridization must the central carbon have? Sketch the bonding π orbitals in allene. What shape do you predict for allene?
Problem 7-62
The heat of hydrogenation for allene (Problem 7-61) to yield propane is −295 kJ/mol, and the heat of hydrogenation for a typical monosubstituted alkene, such as propene, is −125 kJ/mol. Is allene more stable or less stable than you might expect for a diene? Explain.
Problem 7-63
Retin A, or retinoic acid, is a medication commonly used to reduce wrinkles and treat severe acne. How many different isomers arising from E,Z double-bond isomerizations are possible?
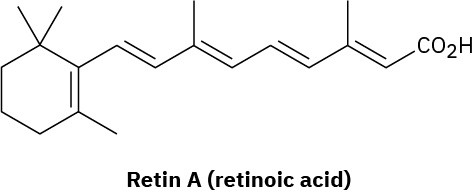
Problem 7-64
Fucoserratene and ectocarpene are sex pheromones produced by marine brown algae. What are their systematic names? (Ectocarpene is difficult; make your best guess, and then check your answer in the Student Solutions Manual.)
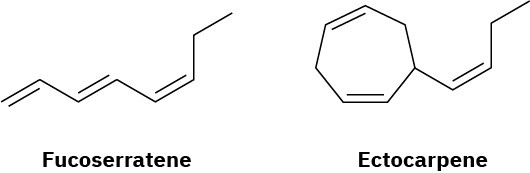
Problem 7-65
tert-Butyl esters [RCO2C(CH3)3] are converted into carboxylic acids (RCO2H) by reaction with trifluoroacetic acid, a reaction useful in protein synthesis (Section 26.7). Assign E,Z designation to the double bonds of both reactant and product in the following scheme, and explain why there is an apparent change in double-bond stereochemistry:
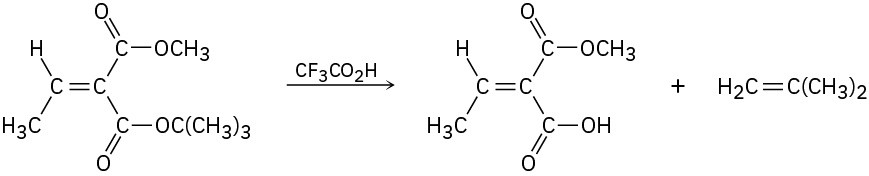
Problem 7-66
Vinylcyclopropane reacts with HBr to yield a rearranged alkyl bromide. Follow the flow of electrons as represented by the curved arrows, show the structure of the carbocation intermediate in brackets, and show the structure of the final product.
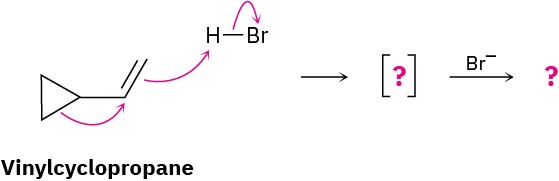
Problem 7-67
Calculate the degree of unsaturation in each of the following formulas: (a)
Cholesterol, C27H46O (b)
DDT, C14H9Cl5
(c)
Prostaglandin E1, C20H34O5 (d)
Caffeine, C8H10N4O2 (e)
Cortisone, C21H28O5 (f)
Atropine, C17H23NO3 Problem 7-68
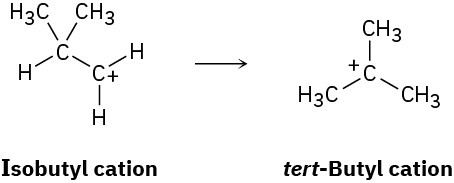
The isobutyl cation spontaneously rearranges to the tert-butyl cation by a hydride shift. Is the rearrangement exergonic or endergonic? Draw what you think the transition state for the hydride shift might look like according to the Hammond postulate.
Problem 7-69
Draw an energy diagram for the addition of HBr to 1-pentene. Let one curve on your diagram show the formation of 1-bromopentane product and another curve on the same diagram show the formation of 2-bromopentane product. Label the positions for all reactants, intermediates, and products. Which curve has the higher-energy carbocation intermediate? Which curve has the higher-energy first transition state?
Problem 7-70
Sketch the transition-state structures involved in the reaction of HBr with 1-pentene (Problem 7-69). Tell whether each structure resembles reactant or product.
Problem 7-71
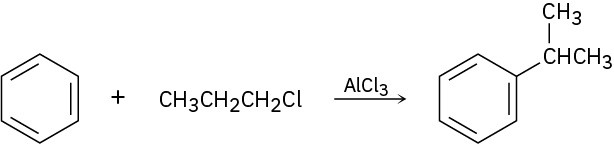
Aromatic compounds such as benzene react with alkyl chlorides in the presence of AlCl3 catalyst to yield alkylbenzenes. This reaction occurs through a carbocation intermediate, formed by reaction of the alkyl chloride with AlCl3 (R−Cl + AlCl3 ⟶ R+ + AlCl4−). How can you explain the observation that reaction of benzene with 1-chloropropane yields isopropylbenzene as the major product?
Problem 7-72
Reaction of 2,3-dimethyl-1-butene with HBr leads to an alkyl bromide, C6H13Br. On treatment of this alkyl bromide with KOH in methanol, elimination of HBr occurs and a hydrocarbon that is isomeric with the starting alkene is formed. What is the structure of this hydrocarbon, and how do you think it is formed from the alkyl bromide?

