6.2 How Organic Reactions Occur: Mechanisms
Having looked at the kinds of reactions that take place, let’s now see how they occur. An overall description of how a reaction occurs is called a reaction mechanism. A mechanism describes in detail exactly what takes place at each stage of a chemical transformation— which bonds are broken and in what order, which bonds are formed and in what order, and what the relative rates are for each step. A complete mechanism must also account for all reactants used and all products formed.
All chemical reactions involve bond-breaking and bond-making. When two molecules come together, react, and yield products, specific bonds in the reactant molecules are broken and specific bonds in the product molecules are formed. Fundamentally, there are two ways in which a covalent two-electron bond can break. A bond can break in an electronically unsymmetrical way so that both bonding electrons remain with one product fragment, leaving the other with a vacant orbital, or a bond can break in an electronically symmetrical way so that one electron remains with each product fragment. The unsymmetrical cleavage is said to be heterolytic, and the symmetrical cleavage is said to be homolytic.

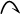 We’ll develop this point in more detail later, but note for now that the movement of two electrons in the unsymmetrical process is indicated using a full-headed curved arrow ( ), whereas the movement of one electron in the symmetrical process is indicated using a half- headed, or “fishhook,” arrow ( ).
We’ll develop this point in more detail later, but note for now that the movement of two electrons in the unsymmetrical process is indicated using a full-headed curved arrow ( ), whereas the movement of one electron in the symmetrical process is indicated using a half- headed, or “fishhook,” arrow ( ).

Just as there are two ways in which a bond can break, there are two ways in which a covalent two-electron bond can form. A bond can form in an electronically unsymmetrical way if both bonding electrons are donated to the new bond by one reactant, or in a symmetrical way if one electron is donated by each reactant.

Processes that involve unsymmetrical bond-breaking and bond-making are called polar reactions. Polar reactions involve species that have an even number of electrons and thus have only electron pairs in their orbitals. Polar processes are by far the more common reaction type in both organic and biological chemistry, and a large part of this book is devoted to their description.
Processes that involve symmetrical bond-breaking and bond-making are called radical reactions. A radical, often called a free radical, is a neutral chemical species that contains an odd number of electrons and thus has a single, unpaired electron in one of its orbitals.
In addition to polar and radical reactions, there is a third, less commonly encountered process called a pericyclic reaction. Rather than explain pericyclic reactions now, though, we’ll look at them more carefully in Chapter 30.

