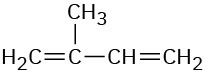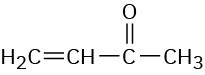14.8 Interpreting Ultraviolet Spectra: The Effect of Conjugation
The wavelength necessary to effect the π → π* transition in a conjugated molecule depends on the energy gap between HOMO and LUMO, which in turn depends on the nature of the conjugated system. Thus, by measuring the UV spectrum of an unknown, we can derive structural information about the nature of any conjugated π electron system present in a molecule.
One of the most important factors affecting the wavelength of UV absorption by a molecule is the extent of conjugation. Molecular orbital calculations show that the energy difference between HOMO and LUMO decreases as the extent of conjugation increases. Thus, 1,3- butadiene absorbs at λmax = 217 nm, 1,3,5-hexatriene absorbs at λmax = 258 nm, and 1,3,5,7- octatetraene absorbs at λmax = 290 nm. (Remember: longer wavelength means lower energy.)
Other kinds of conjugated systems, such as conjugated enones and aromatic rings, also have characteristic UV absorptions that are useful in structure determination. The UV absorption maxima of some representative conjugated molecules are given in Table 14.2.
Table 14.2 Ultraviolet Absorptions of Some Conjugated Molecules
|
Structure |
λmax (nm) |
|
|
2-Methyl-1,3-butadiene |
|
220 |
|
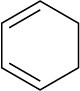
1,3-Cyclohexadiene |
|
256 |
|
1,3,5-Hexatriene |
H2C=CH―CH=CH–CH=CH2 |
258 |
|
1,3,5,7-Octatetraene |
H2C=CH–CH=CH–CH=CH–CH=CH2 |
290 |
|
3-Buten-2-one |
|
219 |
|
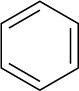
Benzene |
|
203 |
Problem 14-15
Which of the following compounds would you expect to show ultraviolet absorptions in the 200 to 400 nm range?
(a)(b)(f)
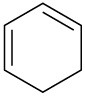
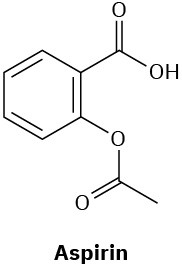
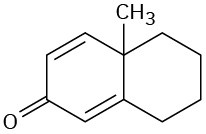 (c)
(c) 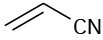 (d)(e)
(d)(e)