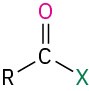Key Terms
- alkoxymercuration
- crown ether
- disulfide (R–S–S–R′)
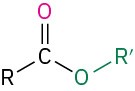
- ether (R–O–R′)
- mercapto group
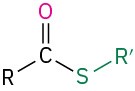
- sulfide (R–S–R′)
- sulfone
- sulfonium ion
- sulfoxide
- thiol
- thiolate ion (RS–)
- Williamson ether synthesis
Preview of Carbonyl Chemistry 18 • Preview of Carbonyl Chemistry 18 • Preview of Carbonyl Chemistry
Carbonyl compounds are everywhere. Most biological molecules contain carbonyl groups, as do most pharmaceutical agents and many of the synthetic chemicals that affect our everyday lives. Citric acid, found in lemons and oranges; acetaminophen, the active ingredient in many over-the-counter headache remedies; and Dacron, the polyester material used in clothing, all contain different kinds of carbonyl groups.
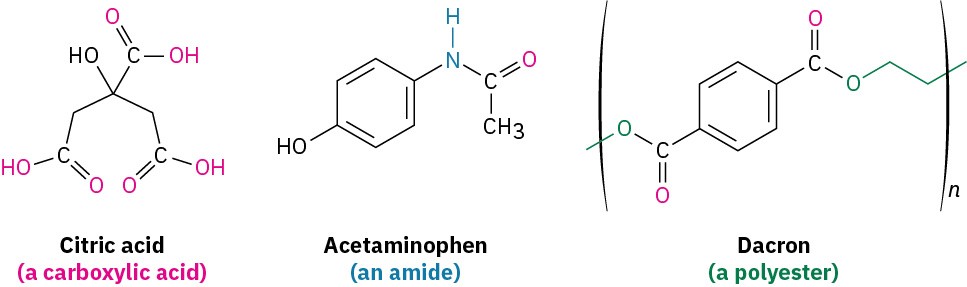
To a great extent, the chemistry of living organisms is the chemistry of carbonyl compounds. Thus, we’ll spend the next five chapters discussing the chemistry of the carbonyl group, C=O (pronounced car-bo-neel). There are many different kinds of carbonyl compounds and many different reactions, but there are only a few fundamental principles that tie the entire field together. The purpose of this brief preview is not to show details of specific reactions but rather to provide a framework for learning carbonyl-group chemistry. Read through this preview now, and return to it on occasion to remind yourself of the larger picture.
I Kinds of Carbonyl Compounds
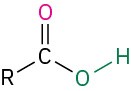
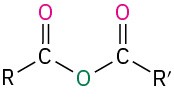
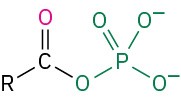
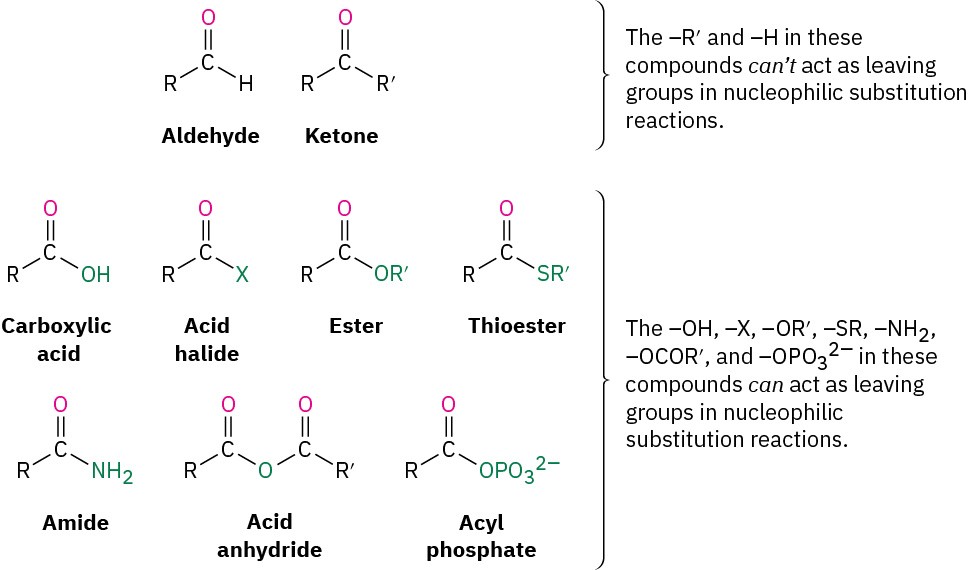
Table 18.2 shows some of the many different kinds of carbonyl compounds. All contain an acyl group (R–C=O) bonded to another substituent. The R part of the acyl group can be practically any organic part/structure, and the other substituent to which the acyl group is bonded might be a carbon, hydrogen, oxygen, halogen, nitrogen, or sulfur.
Table 18.2 Some Types of Carbonyl Compounds
|
Name |
General formula |
Name ending |
|
Aldehyde |
|
-al |
|
Ketone |
|
-one |
|
Carboxylic acid |
|
-oic acid |
|
General formula |
Name ending |
|
|
Acid halide |
|
-yl or -oyl halide |
|
Acid anhydride |
|
-oic anhydride |
|
Acyl phosphate |
|
-yl phosphate |
|
Ester |
|
–oate |
|
Lactone (cyclic ester) |
|
None |
|
Thioester |
|
–thioate |
|
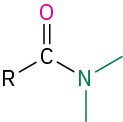
Amide |
|
–amide |
|
Lactam (cyclic amide) |
|
None |
It’s useful to classify carbonyl compounds into two categories based on the kinds of chemistry they undergo. In one category are aldehydes and ketones; in the other are carboxylic acids and their derivatives. The acyl group in an aldehyde or ketone is bonded to an atom (H or C, respectively) that can’t stabilize a negative charge and therefore can’t act as a leaving group in a nucleophilic substitution reaction. The acyl group in a carboxylic acid or its derivative, however, is bonded to an atom (oxygen, halogen, sulfur, nitrogen) that can stabilize a negative charge and therefore can act as a leaving group in a nucleophilic substitution reaction.
- Nature of the Carbonyl Group
The carbon–oxygen double bond of a carbonyl group is similar in many respects to the carbon–carbon double bond of an alkene. The carbonyl carbon atom is sp2-hybridized and forms three σ bonds. The fourth valence electron remains in a carbon p orbital and forms a π bond to oxygen by overlapping with an oxygen p orbital. The oxygen atom also has two nonbonding pairs of electrons, which occupy its remaining two orbitals.
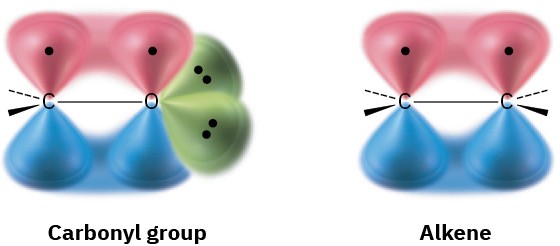
Like alkenes, carbonyl compounds are planar about the double bond and have bond angles of approximately 120°. Figure 18.8 shows the structure of acetaldehyde and indicates its bond lengths and angles. As you might expect, the carbon–oxygen double bond is both shorter (122 pm versus 143 pm) and stronger [732 kJ/mol (175 kcal/mol) versus 385 kJ/mol (92 kcal/mol)] than a C–O single bond.
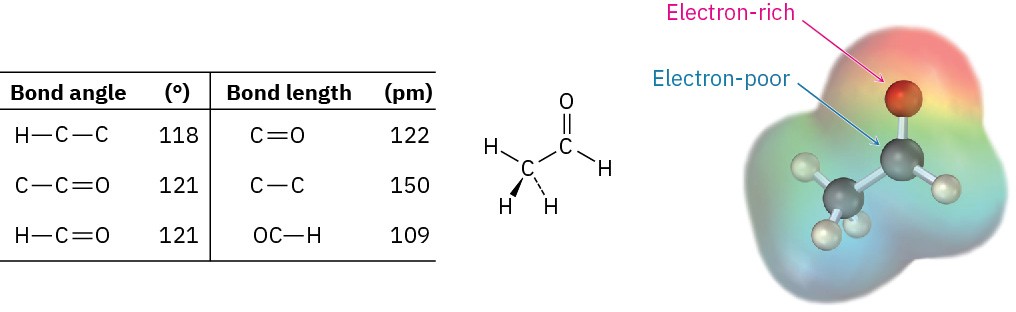
Figure 18.8 Structure of acetaldehyde.
As indicated by the electrostatic potential map in Figure 18.8, the carbon–oxygen double bond is strongly polarized because of the high electronegativity of oxygen relative to carbon. Thus, the carbonyl carbon atom carries a partial positive charge, is an electrophilic (Lewis acidic) site, and reacts with nucleophiles. Conversely, the carbonyl oxygen atom carries a partial negative charge, is a nucleophilic (Lewis basic) site, and reacts with electrophiles. We’ll see in the next five chapters that the majority of carbonyl-group reactions can be rationalized by simple polarity arguments.
- General Reactions of Carbonyl Compounds
Both in the laboratory and in living organisms, most reactions of carbonyl compounds take place by one of four general mechanisms: nucleophilic addition, nucleophilic acyl substitution, alpha substitution, and carbonyl condensation. These mechanisms have many variations, just as alkene electrophilic addition reactions and SN2 reactions do, but the variations are much easier to learn when the fundamental features of the mechanisms are made clear. Let’s see what the four mechanisms are and what kinds of chemistry carbonyl compounds undergo.
Nucleophilic Addition Reactions of Aldehydes and Ketones (Chapter 19)
The most common reaction of aldehydes and ketones is the nucleophilic addition reaction, in which a nucleophile, :Nu–, adds to the electrophilic carbon of the carbonyl group. Because the nucleophile uses an electron pair to form a new bond to carbon, two electrons from the carbon–oxygen double bond must move toward the electronegative oxygen atom to give an alkoxide anion. The carbonyl carbon rehybridizes from sp2 to sp3 during the reaction, and the alkoxide ion product therefore has tetrahedral geometry.
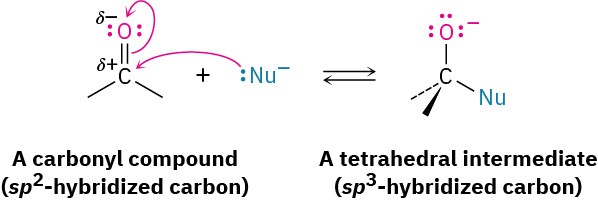
Once formed, and depending on the nature of the nucleophile, the tetrahedral alkoxide intermediate can undergo one of two further reactions, as shown in Figure 18.9. Often, the tetrahedral alkoxide intermediate is simply protonated by water or acid to form an alcohol product. Alternatively, the tetrahedral intermediate can be protonated and expel the oxygen to form a new double bond between the carbonyl carbon and the nucleophile. We’ll study both processes in detail in Chapter 19.
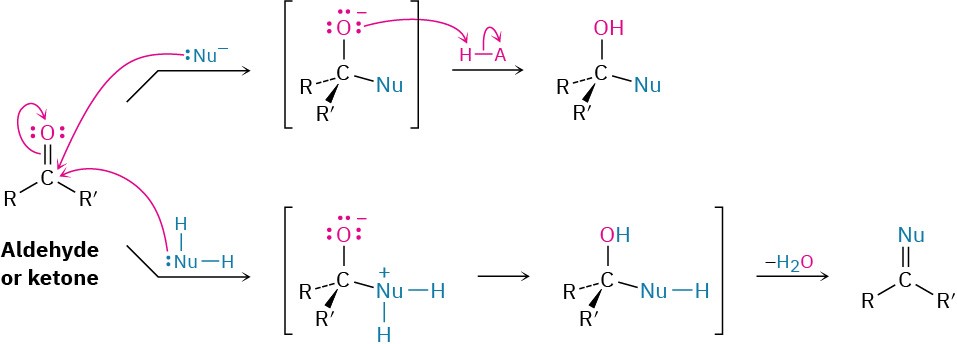
Figure 18.9 The addition reaction of an aldehyde or a ketone with a nucleophile. Depending on the nucleophile, either an alcohol or a compound with a C═Nu double bond is formed.
FORMATION OF AN ALCOHOL
The simplest reaction of a tetrahedral alkoxide intermediate is protonation to yield an alcohol. We’ve already seen two examples of this kind of process during reduction of aldehydes and ketones with hydride reagents such as NaBH4 and LiAlH4 (Section 17.4) and during Grignard reactions (Section 17.5). During a reduction, the nucleophile that adds to the carbonyl group is a hydride ion, H:–, while during a Grignard reaction, the nucleophile is a carbanion, R3C:–.
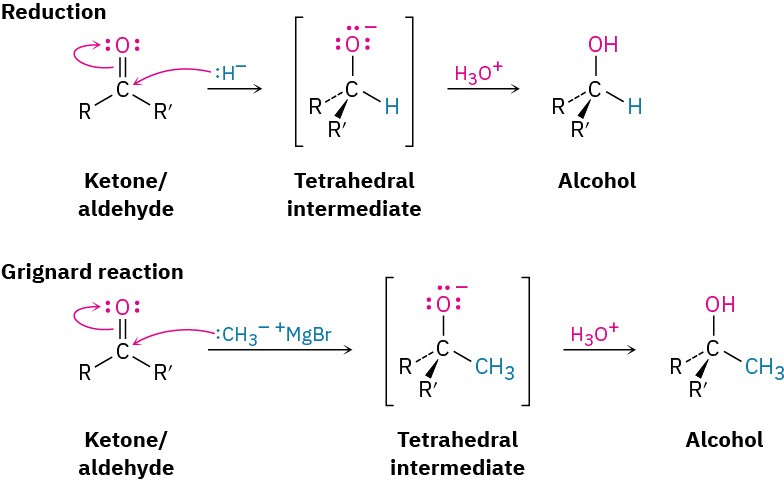
FORMATION OF C=Nu
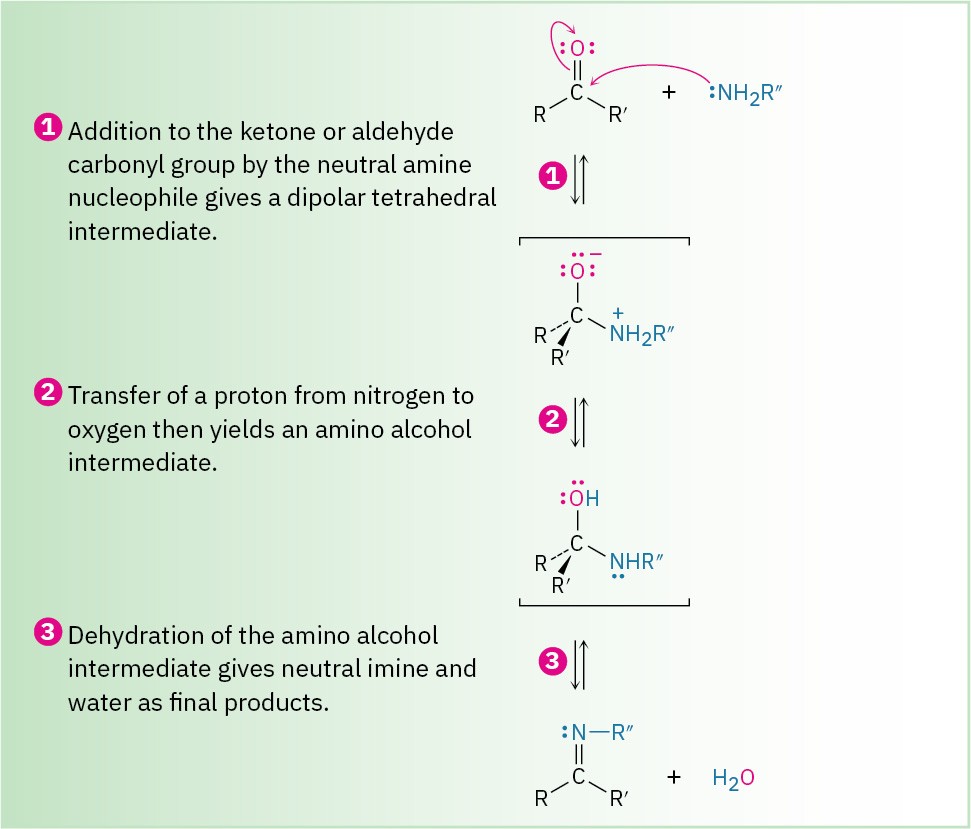
The second mode of nucleophilic addition, which often occurs with amine nucleophiles, involves elimination of oxygen and formation of a C=Nu double bond. For example, aldehydes and ketones react with primary amines, RNH2, to form imines, R!C=NR′. These reactions use the same kind of tetrahedral intermediate as that formed during hydride reduction and Grignard reaction, but the initially formed alkoxide ion is not isolated.
Instead, it is protonated and then loses water to form an imine, as shown in Figure 18.10. Figure 18.10 MECHANISM
Formation of an imine, R2C=NR′, by reaction of an amine with an aldehyde or a ketone.
Nucleophilic Acyl Substitution Reactions of Carboxylic Acid Derivatives (Chapter 21)
The second fundamental reaction of carbonyl compounds, nucleophilic acyl substitution, is related to the nucleophilic addition reaction just discussed but occurs only with carboxylic acid derivatives rather than with aldehydes and ketones. When the carbonyl group of a carboxylic acid derivative reacts with a nucleophile, addition occurs in the usual way, but the initially formed tetrahedral alkoxide intermediate is not isolated. Because carboxylic acid derivatives have a leaving group bonded to the carbonyl-group carbon, the tetrahedral intermediate can react further by expelling the leaving group and forming a new carbonyl compound:
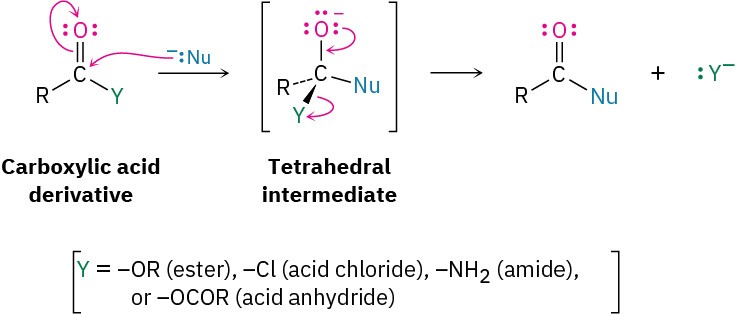
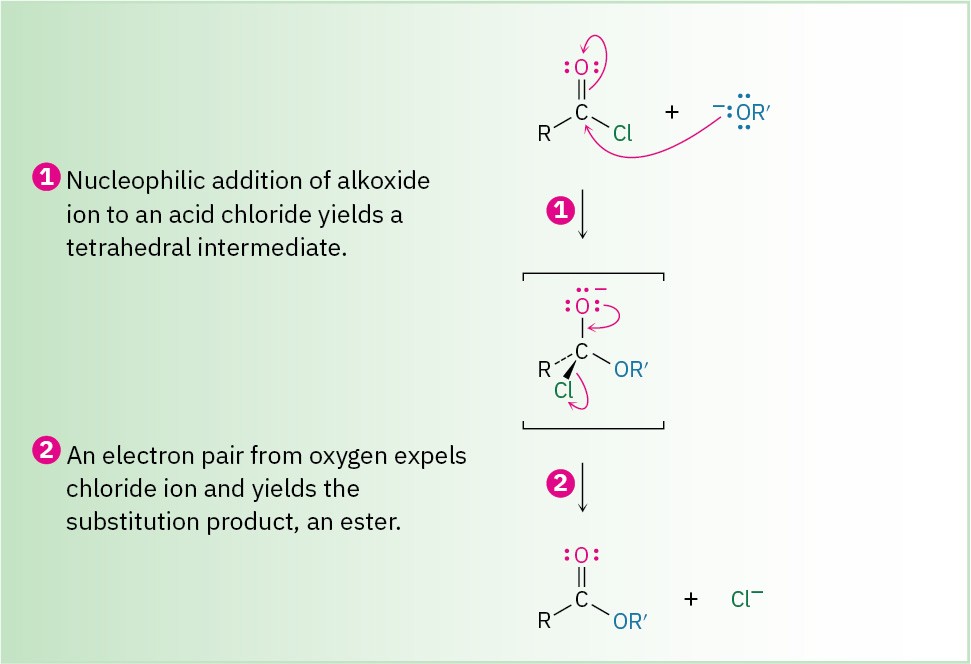
The net effect of nucleophilic acyl substitution is the replacement of the leaving group by the entering nucleophile. We’ll see in Chapter 21, for instance, that acid chlorides are rapidly converted into esters by treatment with alkoxide ions (Figure 18.11).
Figure 18.11 MECHANISM
Nucleophilic acyl substitution of an acid chloride with an alkoxide ion yields an ester.
Alpha-Substitution Reactions (Chapter 22)
The third major reaction of carbonyl compounds, alpha substitution, occurs at the position next to the carbonyl group—the alpha (α) position. This reaction results in the substitution of an α hydrogen by an electrophile through the formation of an intermediate enol or enolate ion:
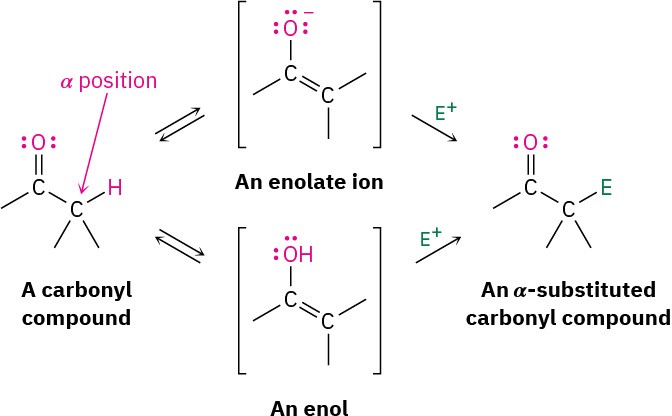
For reasons that we’ll explore in Chapter 22, the presence of a carbonyl group renders the hydrogens on the α carbon acidic. Carbonyl compounds therefore react with strong base to yield enolate ions.
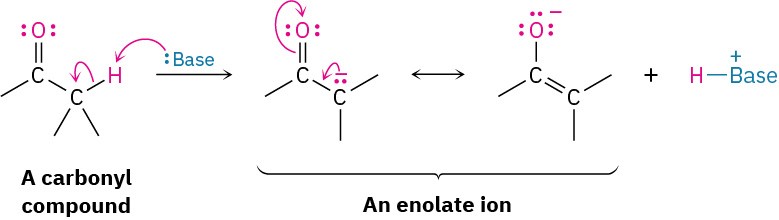
Because they’re negatively charged, enolate ions act as nucleophiles and undergo many of the reactions we’ve already studied. For example, enolates react with primary alkyl halides in the SN2 reaction. The nucleophilic enolate ion displaces halide ion, and a new C–C bond forms:
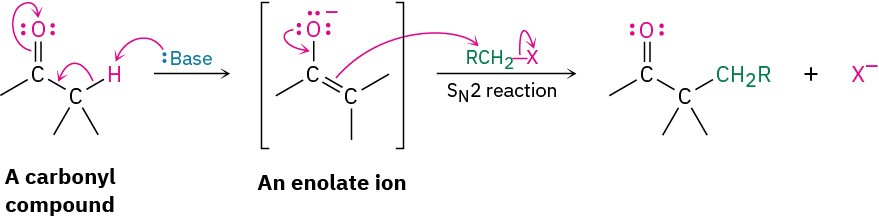
The SN2 alkylation reaction between an enolate ion and an alkyl halide is a powerful method for making C–C bonds, thereby building up larger molecules from smaller precursors. We’ll study the alkylation of many kinds of carbonyl compounds in Chapter 22.
Carbonyl Condensation Reactions (Chapter 23)
The fourth and last fundamental reaction of carbonyl groups, carbonyl condensation, takes place when two carbonyl compounds react with each other. When acetaldehyde is treated with base, for instance, two molecules combine to yield the hydroxy aldehyde product known as aldol (aldehyde + alcohol):
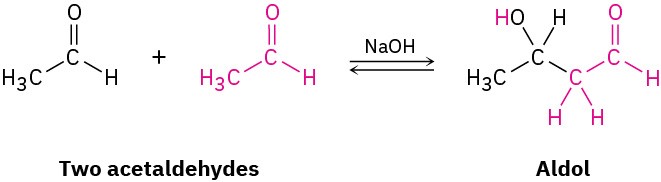
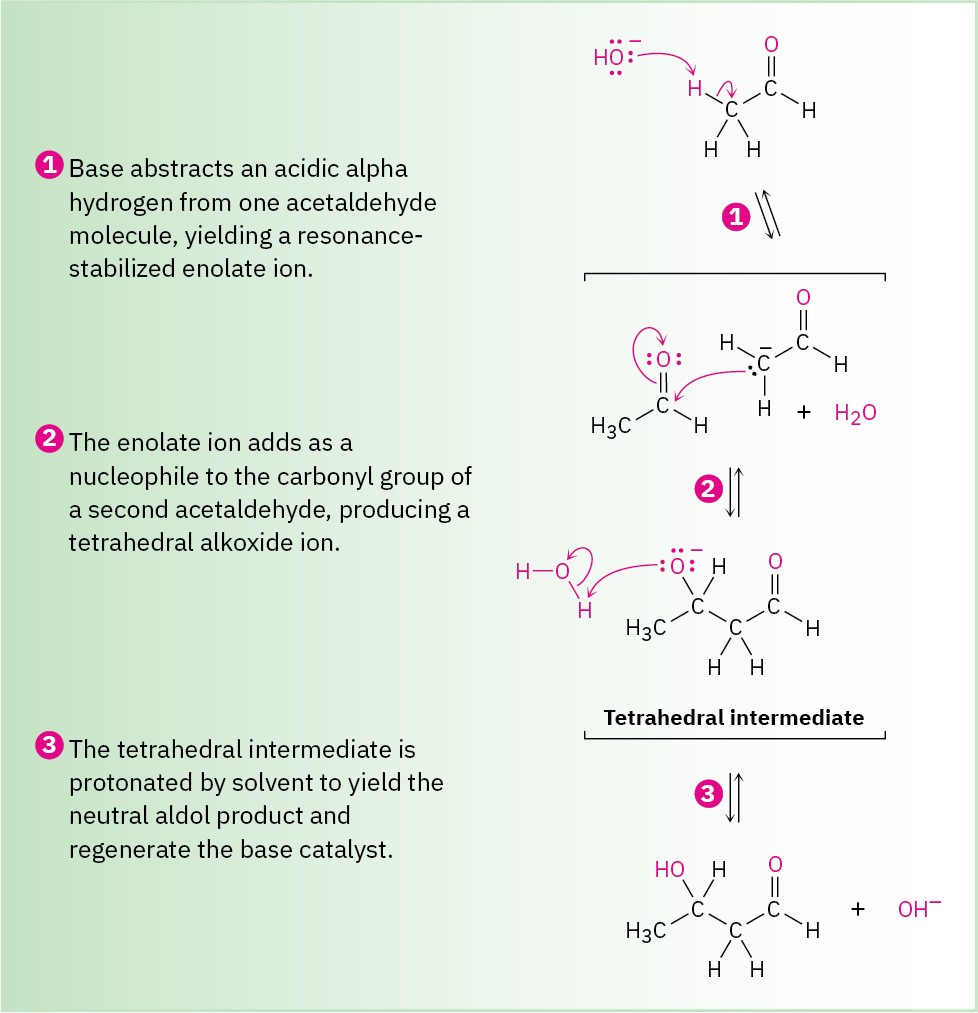
Although carbonyl condensation appears to be different from the three processes already discussed, it’s actually quite similar. A carbonyl condensation reaction is simply a combination of a nucleophilic addition step and an α-substitution step. The initially formed enolate ion of one acetaldehyde molecule acts as a nucleophile and adds to the carbonyl group of another acetaldehyde molecule, as shown in Figure 18.12.
Figure 18.12 MECHANISM
A carbonyl condensation reaction between two molecules of acetaldehyde yields a hydroxy aldehyde product.
- Summary
To a great extent, the chemistry of living organisms is the chemistry of carbonyl compounds. We have not looked at the details of specific carbonyl reactions in this short preview but rather have laid the groundwork for the next five chapters. All the carbonyl- group reactions we’ll be studying in Chapters 19 through 23 fall into one of the four fundamental categories discussed in this preview. Knowing where we’ll be heading should help you keep matters straight in understanding this most important of all functional groups.
Problem 18-69
Judging from the following electrostatic potential maps, which kind of carbonyl compound has the more electrophilic carbonyl carbon atom, a ketone or an acid chloride? Which has the more nucleophilic carbonyl oxygen atom? Explain.
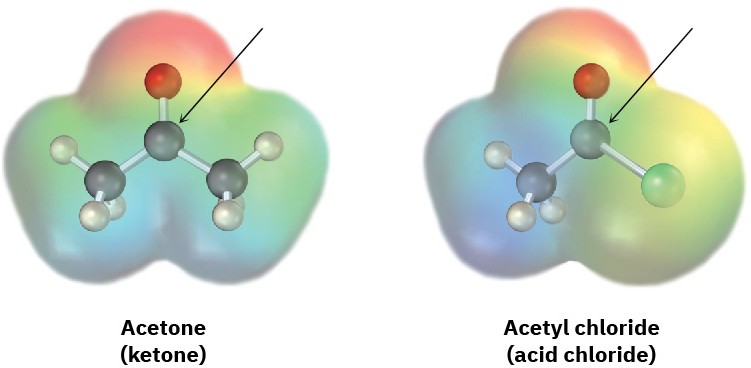
Problem 18-70
Predict the product formed by nucleophilic addition of cyanide ion (CN–) to the carbonyl group of acetone, followed by protonation to give an alcohol:
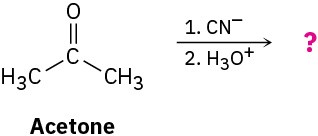
Problem 18-71
Identify each of the following reactions as a nucleophilic addition, nucleophilic acyl substitution, an α substitution, or a carbonyl condensation:
(a)
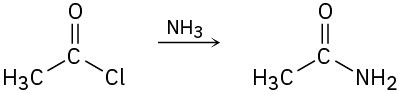
(b)
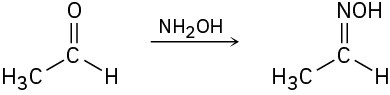
(c)
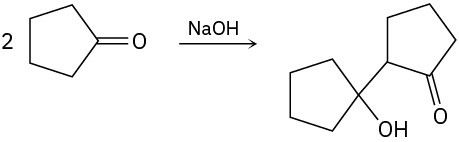
Summary of Reactions 18 • Summary of Reactions 18 • Summary of Reactions
- Synthesis of ethers (Section 18.2)
- Williamson ether synthesis

- Alkoxymercuration/demercuration
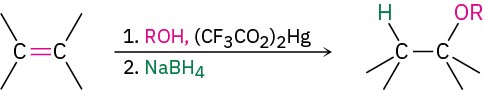
- Reactions of ethers
- Cleavage by HBr or HI (Section 18.3)
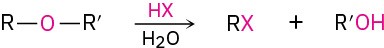
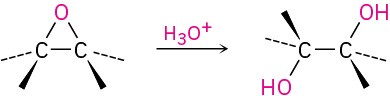
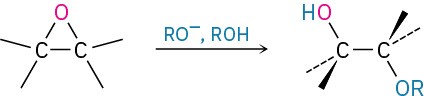
- Acid-catalyzed epoxide opening (Section 18.5)
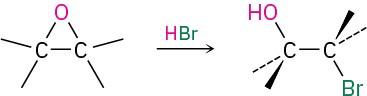
- Base-catalyzed epoxide opening (Section 18.5)
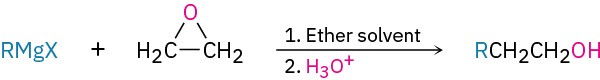
- Synthesis of thiols (Section 18.7)
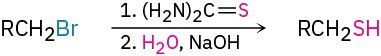
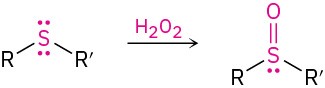
- Oxidation of thiols to disulfides (Section 18.7)
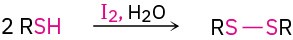
- Synthesis of sulfides (Section 18.7)

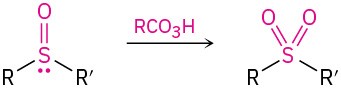
- Oxidation of sulfides to sulfoxides and sulfones (Section 18.7)