6.1 Kinds of Organic Reactions
Organic chemical reactions can be organized broadly in two ways—by what kinds of reactions occur and by how those reactions occur. Let’s look first at the kinds of reactions that take place. There are four general types of organic reactions: additions, eliminations, substitutions, and rearrangements.
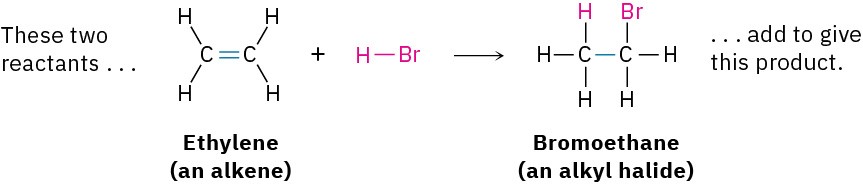
- Addition reactions occur when two reactants add together to form a single product with no atoms “left over.” An example that we’ll be studying soon is the reaction of an alkene, such as ethylene, with HBr to yield an alkyl bromide.
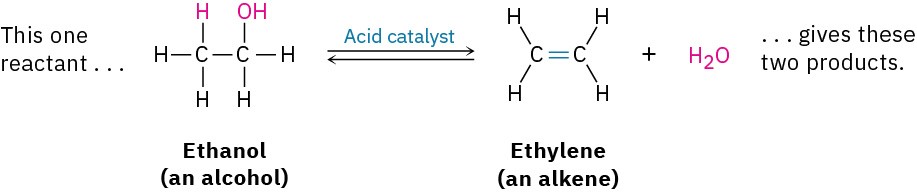
- Elimination reactions are, in a sense, the opposite of addition reactions. They occur when a single reactant splits into two products, often with the formation of a small molecule such as water or HBr. An example is the acid-catalyzed reaction of an alcohol to yield water and an alkene.
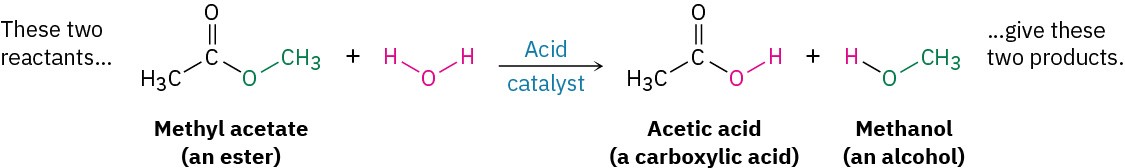
- Substitution reactions occur when two reactants exchange parts to give two new products. An example is the reaction of an ester such as methyl acetate with water to yield a carboxylic acid plus an alcohol. Similar reactions occur in many biological pathways, including the metabolism of dietary fats.
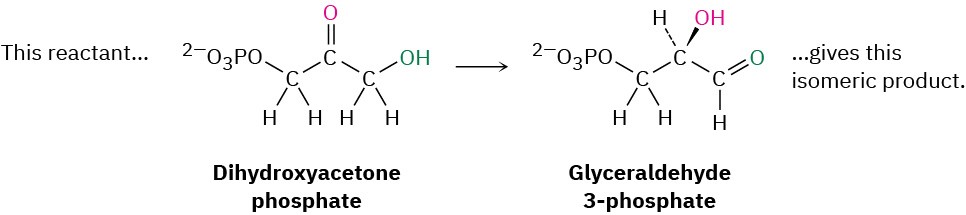
- Rearrangement reactions occur when a single reactant undergoes a reorganization of bonds and atoms to yield an isomeric product. An example is the conversion of dihydroxyacetone phosphate into its constitutional isomer glyceraldehyde 3- phosphate, a step in the glycolysis pathway by which carbohydrates are metabolized.
Problem 6-1
Classify each of the following reactions as an addition, elimination, substitution, or rearrangement:
(a)
CH3Br + KOH ⟶ CH3OH + KBr
(b)
CH3CH2Br ⟶ H2C=CH2 + HBr
(c)
H2C=CH2 + H2 ⟶ CH3CH3

