10.1 Names and Structures of Alkyl Halides
Although commonly called alkyl halides, halogen-substituted alkanes are named systematically as haloalkanes (Section 3.4), treating the halogen as a substituent on a parent alkane chain. There are three steps:
STEP 1
Find the longest chain, and name it as the parent. If a double or triple bond is present, the parent chain must contain it.
STEP 2
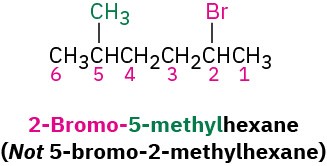
Number the carbons of the parent chain beginning at the end nearer the first substituent, whether alkyl or halo. Assign each substituent a number according to its position on the chain.

If different halogens are present, number each one and list them in alphabetical order when writing the name.
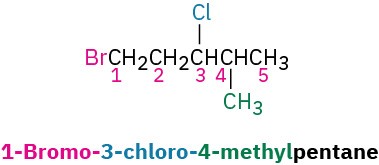
STEP 3
If the parent chain can be properly numbered from either end by step 2, begin at the end nearer the substituent that has alphabetical precedence.
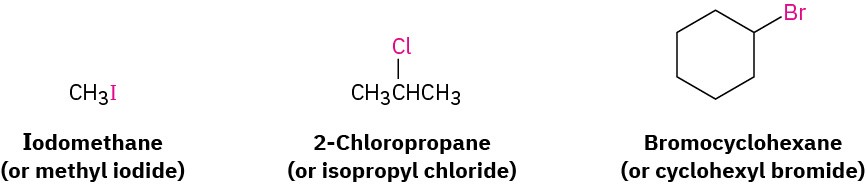
In addition to their systematic names, many simple alkyl halides are also named by identifying first the alkyl group and then the halogen. For example, CH3I can be called either iodomethane or methyl iodide. Such names are well entrenched in the chemical literature and in daily usage, but they won’t be used in this book.
Halogens increase in size going down the periodic table, so the lengths of the corresponding carbon–halogen bonds increase accordingly (Table 10.1). In addition, C−X bond strengths decrease going down the periodic table. As we’ve been doing thus far, we’ll continue using an X to represent any of the halogens F, Cl, Br, or I.
Table 10.1 A Comparison of the Halomethanes
|
Bond length (pm) |
Bond strength |
Dipole moment (D) |
||
|
(kJ/mol) |
(kcal/mol) |
|||
|
CH3F |
139 |
460 |
110 |
1.85 |
|
CH3Cl |
178 |
350 |
84 |
1.87 |
|
CH3Br |
193 |
294 |
70 |
1.81 |
|
CH3I |
214 |
239 |
57 |
1.62 |
In our discussion of bond polarity in functional groups in Section 6.3, we noted that halogens are more electronegative than carbon. The C−X bond is therefore polar, with the carbon atom bearing a slight positive charge (δ+) and the halogen a slight negative charge (δ−). This polarity results in a dipole moment for all the halomethanes (Table 10.1) and implies that the alkyl halide C−X carbon atom should behave as an electrophile in polar reactions. We’ll soon see that this is indeed the case.
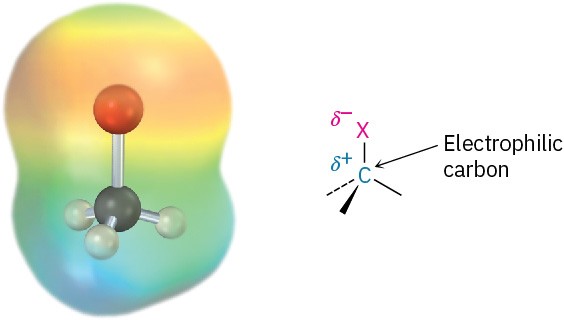
Problem 10-1
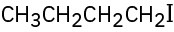 Give IUPAC names for the following alkyl halides: (a)
Give IUPAC names for the following alkyl halides: (a)
(b)
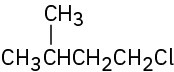
(c)
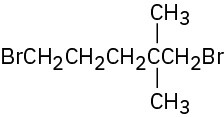
(d)
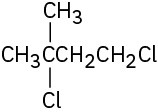
(e)
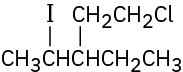
(f)
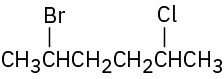
Problem 10-2
Draw structures corresponding to the following IUPAC names: (a)
- Chloro-3,3-dimethylhexane (b)
3,3-Dichloro-2-methylhexane (c)
- Bromo-3-ethylpentane (d)
1,1-Dibromo-4-isopropylcyclohexane (e)
- sec-Butyl-2-chlorononane (f)
1,1-Dibromo-4-tert-butylcyclohexane

