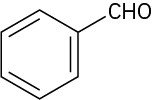
19.1 Naming Aldehydes and Ketones
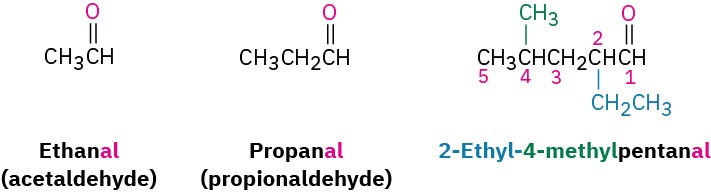
Aldehydes are named by replacing the terminal –e of the corresponding alkane name with – al. The parent chain must contain the –CHO group, and the –CHO carbon is numbered as carbon 1. Note in the following examples that the longest chain in 2-ethyl-4- methylpentanal is actually a hexane, but this chain does not include the –CHO group and thus is not the parent.
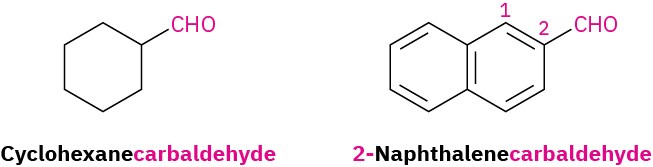
For cyclic aldehydes in which the –CHO group is directly attached to a ring, the ring name followed by carbaldehyde is used.
A few simple and well-known aldehydes have common names that are recognized by IUPAC. Several that you might encounter are listed in Table 19.1.
Table 19.1 Common Names of Some Simple Aldehydes
|
Common name |
Systematic name |
|
|
HCHO |
Formaldehyde |
Methanal |
|
CH3CHO |
Acetaldehyde |
Ethanal |
|
H!C═CHCHO |
Acrolein |
Propenal |
|
CH“CH═CHCHO |
Crotonaldehyde |
2-Butenal |
|
|
Benzaldehyde |
Benzenecarbaldehyde |
Ketones are named by replacing the terminal –e of the corresponding alkane name with – one. The parent chain is the longest one that includes the ketone group, and the numbering begins at the end nearer the carbonyl carbon. As with alkenes (Section 7.3) and alcohols (Section 17.1), the locant is placed before the parent name using older rules but before the suffix with the newer IUPAC guidelines. For example:
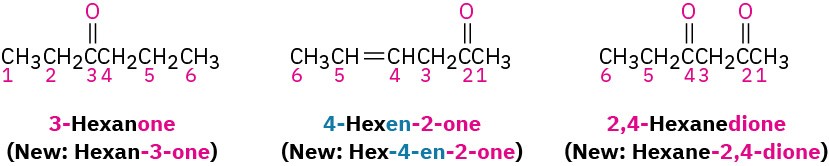
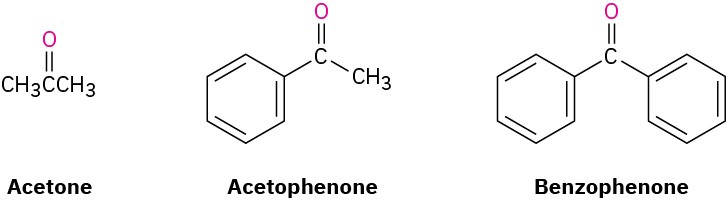
A few ketones are allowed by IUPAC to retain their common names.
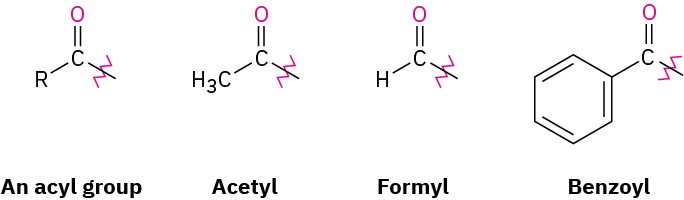
When it’s necessary to refer to the R–C═O group as a substituent, the name acyl (a-sil) group is used and the name ending –yl is attached. Thus, –COCH3 is an acetyl group, –CHO is a formyl group, –COAr is an aroyl group, and –COC6H5 is a benzoyl group.
If other functional groups are present and the double-bonded oxygen is considered a substituent on a parent chain, the prefix oxo– is used. For example:
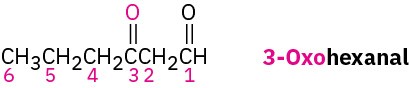
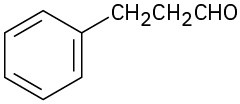
Problem 19-1
 Name the following aldehydes and ketones: (a)
Name the following aldehydes and ketones: (a)
(b)
(c)
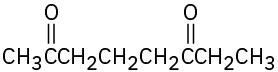
(d)
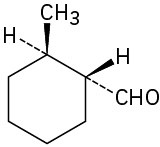
(e)
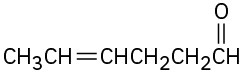
(f)
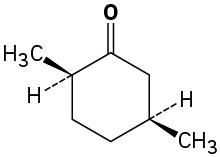
Problem 19-2
Draw structures corresponding to the following names: (a)
- Methylbutanal (b)
- Chloro-2-pentanone (c) Phenylacetaldehyde (d)
cis-3-tert-Butylcyclohexanecarbaldehyde (e)
3-Methyl-3-butenal (f)
2-(1-Chloroethyl)-5-methylheptanal