16.8 Oxidation of Aromatic Compounds
Oxidation of Alkyl Side Chains
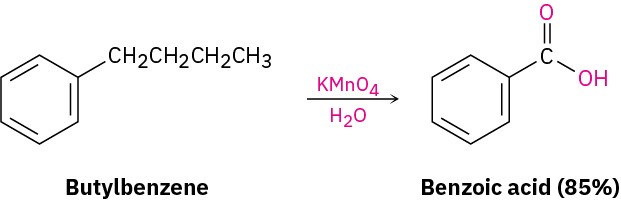
Despite its unsaturation, the benzene ring is inert to strong oxidizing agents such as KMnO4, which will cleave alkene carbon–carbon bonds (Section 8.8). It turns out, however, that the presence of the aromatic ring has a dramatic effect on the reactivity of alkyl side chains. These side chains react rapidly with oxidizing agents and are converted into carboxyl groups, –CO2H.The net effect is conversion of an alkylbenzene into a benzoic acid, Ar–R → Ar– CO!H. Butylbenzene is oxidized by aqueous KMnO4 to give benzoic acid, for instance.
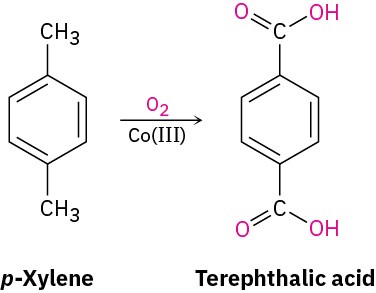
A similar oxidation is employed industrially for the preparation of the terephthalic acid used in the production of polyester fibers. Worldwide, approximately 118 million tons per year of terephthalic acid is produced by oxidation of p-xylene, using air as the oxidant and Co(III) salts as catalyst.
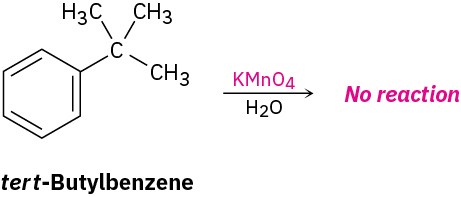
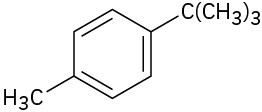
The mechanism of side-chain oxidation is complex and involves reaction of C–H bonds at the position next to the aromatic ring to form intermediate benzylic radicals. tert– Butylbenzene has no benzylic hydrogens, however, and is therefore inert.
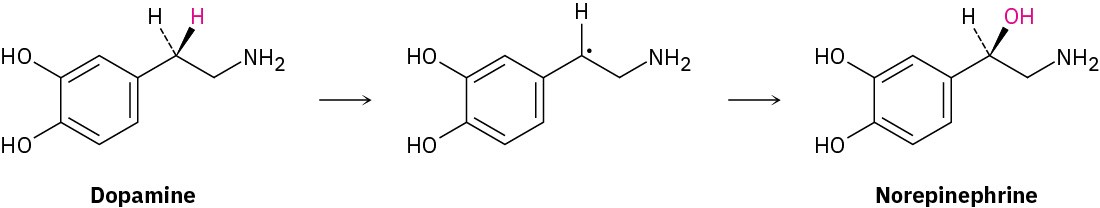
Analogous side-chain oxidations occur in various biosynthetic pathways. The neurotransmitter norepinephrine, for instance, is biosynthesized from dopamine by a benzylic hydroxylation reaction. The process is catalyzed by the copper-containing enzyme
dopamine β-monooxygenase and occurs by a radical mechanism. A copper–oxygen species in the enzyme first abstracts the pro-R benzylic hydrogen to give a radical, and a hydroxyl is then transferred from copper to carbon.
Problem 16-18
What aromatic products would you obtain from the KMnO4 oxidation of the following substances?
(a)
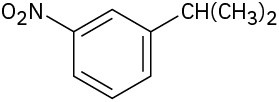
(b)
Bromination of Alkylbenzene Side Chains
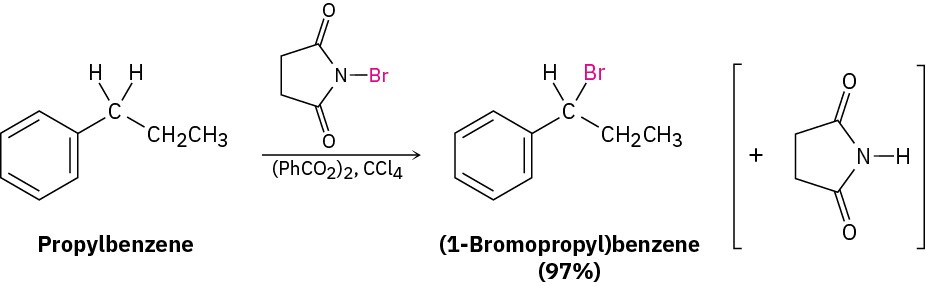
Side-chain bromination at the benzylic position occurs when an alkylbenzene is treated with N-bromosuccinimide (NBS). For example, propylbenzene gives (1- bromopropyl)benzene in 97% yield on reaction with NBS in the presence of benzoyl peroxide, (PhCO2)2, as a radical initiator. Bromination occurs exclusively in the benzylic position next to the aromatic ring and does not give a mixture of products.
The mechanism of benzylic bromination is similar to that discussed in Section 10.3 for allylic bromination of alkenes. Abstraction of a benzylic hydrogen atom first generates an intermediate benzylic radical, which then reacts with Br2 in step 2 to yield product and a Br ⋅ radical, which cycles back into the reaction to carry on the chain shown below as a summary. The Br2 needed for reaction with the benzylic radical is produced in step 3 by a concurrent reaction of HBr with NBS.
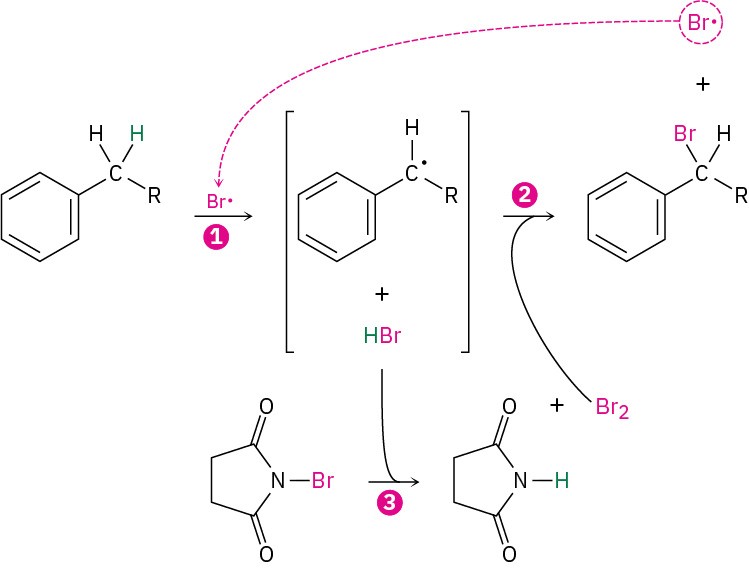
Reaction occurs exclusively at the benzylic position because the benzylic radical intermediate is stabilized by resonance. Figure 16.21 shows how the benzyl radical is stabilized by overlap of its p orbital with the ringed π electron system.
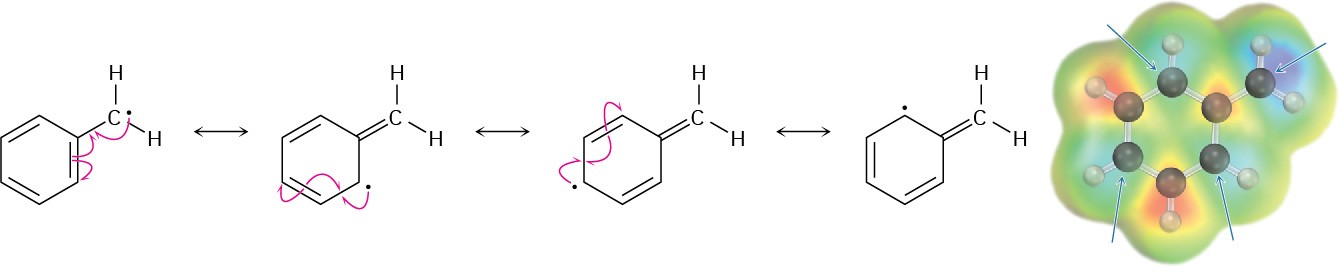
Figure 16.21 A resonance-stabilized benzylic radical. The spin-density surface shows that the unpaired electron is shared by the ortho and para carbons of the ring.
Problem 16-19
Refer to Table 6.3 for a quantitative idea of the stability of a benzyl radical. How much more stable (in kJ/mol) is the benzyl radical than a primary alkyl radical? How does a benzyl radical compare in stability to an allyl radical?
Problem 16-20
Styrene, the simplest alkenylbenzene, is prepared commercially for use in plastics manufacture by catalytic dehydrogenation of ethylbenzene. How might you prepare styrene from benzene using reactions you’ve studied?