30.4 Photochemical Electrocyclic Reactions
We noted previously that photochemical electrocyclic reactions take a different stereochemical course than their thermal counterparts, and we can now explain this difference. Ultraviolet irradiation of a polyene causes an excitation of one electron from the ground-state HOMO to the ground-state LUMO, thus changing their symmetries. But because electronic excitation changes the symmetries of HOMO and LUMO, it also changes the reaction stereochemistry. (2E,4E)-2,4-Hexadiene, for instance, undergoes photochemical cyclization by a disrotatory path, whereas the thermal reaction is conrotatory. Similarly, (2E,4Z,6E)-2,4,6-octatriene undergoes photochemical cyclization by a conrotatory path, whereas the thermal reaction is disrotatory (Figure 30.8).
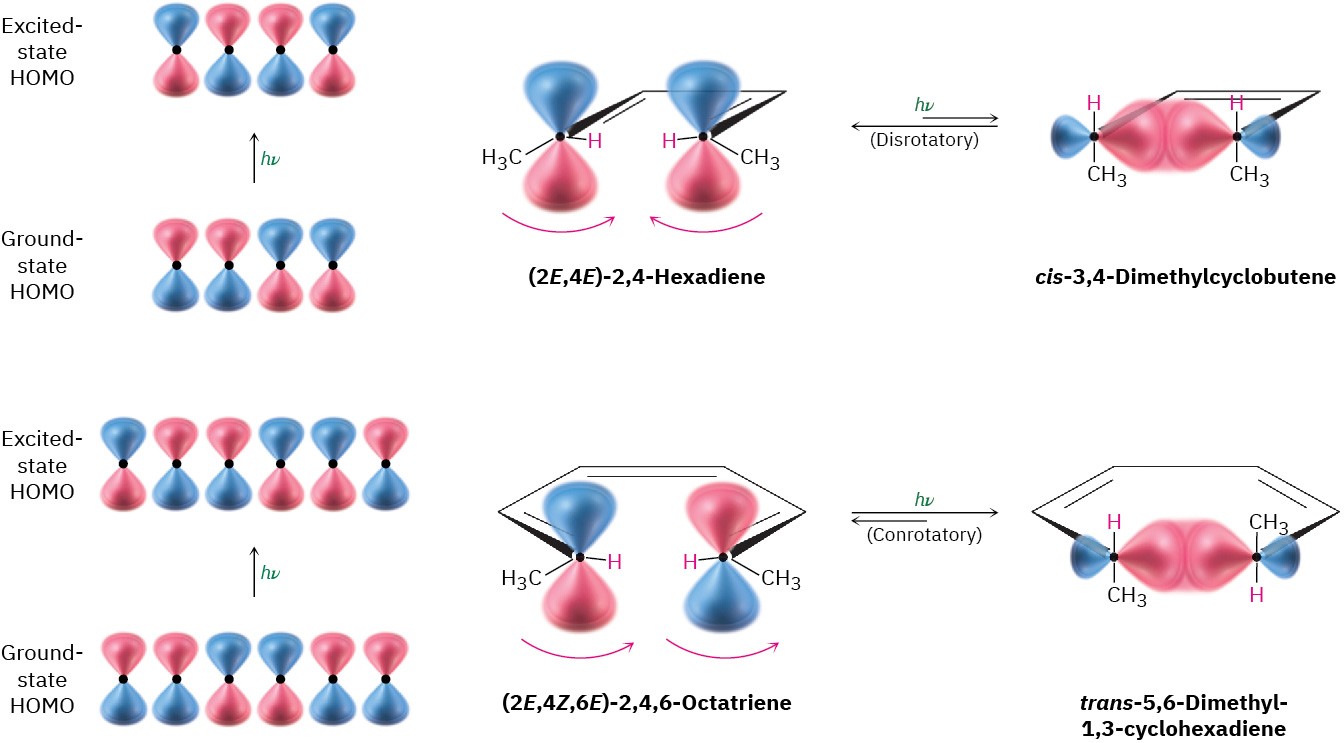
Figure 30.8 Photochemical cyclizations of conjugated dienes and trienes. The two processes occur with different stereochemistry because of their different orbital symmetries.
Thermal and photochemical electrocyclic reactions always take place with opposite stereochemistry because the symmetries of the frontier orbitals are always different. Table
30.1 gives some simple rules that make it possible to predict the stereochemistry of electrocyclic reactions.
Table 30.1 Stereochemical Rules for Electrocyclic Reactions
|
Electron pairs (double bonds) |
Thermal reaction |
Photochemical reaction |
|
Thermal reaction |
Photochemical reaction |
|
|
Even number |
Conrotatory |
Disrotatory |
|
Odd number |
Disrotatory |
Conrotatory |
Problem 30-4
What product would you expect to obtain from the photochemical cyclization of (2E,4Z,6E)- 2,4,6-octatriene? Of (2E,4Z,6Z)-2,4,6-octatriene?

