2.2 Polar Covalent Bonds and Dipole Moments
Just as individual bonds are often polar, molecules as a whole are often polar as well. Molecular polarity results from the vector summation of all individual bond polarities and lone-pair contributions in the molecule. As a practical matter, strongly polar substances are often soluble in polar solvents like water, whereas less polar substances are insoluble in water.
Net polarity is measured by a quantity called the dipole moment and can be thought of in the following way: assume that there is a center of mass of all positive charges (nuclei) in a molecule and a center of mass of all negative charges (electrons). If these two centers don’t coincide, then the molecule has a net polarity.
The dipole moment, μ (lowercase Greek letter mu), is defined as the magnitude of the charge Q at either end of the molecular dipole times the distance r between the charges, μ = Q × r. Dipole moments are expressed in debyes (D), where 1 D = 3.336 × 10–30 coulomb meters (C · m) in SI units. For example, the unit charge on an electron is 1.60 × 10–19 C. Thus, if one positive charge and one negative charge are separated by 100 pm (a bit less than the length of a typical covalent bond), the dipole moment is 1.60 × 10–29 C · m, or 4.80 D.
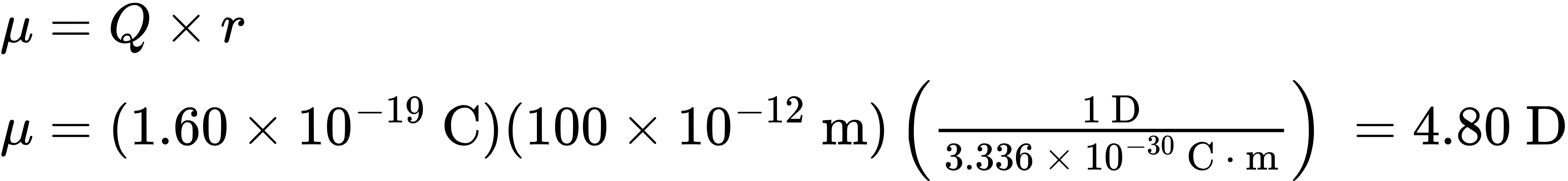
Dipole moments for some common substances are given in Table 2.1. Of the compounds shown in the table, sodium chloride has the largest dipole moment (9.00 D) because it is ionic. Even small molecules like water (μ = 1.85 D), methanol (CH3OH; μ = 1.70 D), and ammonia (μ = 1.47 D), have substantial dipole moments, however, both because they contain strongly electronegative atoms (oxygen and nitrogen) and because all three molecules have lone-pair electrons. The lone-pair electrons on oxygen and nitrogen stick out into space away from the positively charged nuclei, giving rise to a considerable charge separation and making a large contribution to the dipole moment.
Table 2.1 Dipole Moments of Some Compounds
|
Compound |
Dipole moment (D) |
Compound |
Dipole moment (D) |
|
NaCl |
9.00 |
NH3 |
1.47 |
|
CH2O |
2.33 |
CH3NH2 |
1.31 |
|
CH3Cl |
1.87 |
CO2 |
0 |
|
H2O |
1.85 |
CH4 |
0 |
|
CH3OH |
1.70 |
CH3CH3 |
0 |
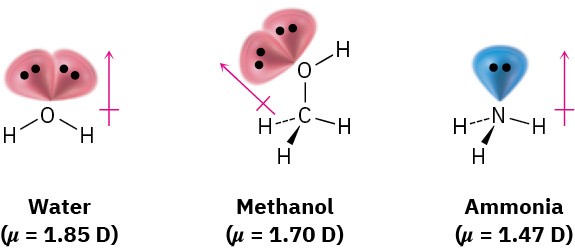
In contrast with water, methanol, and ammonia, molecules such as carbon dioxide, methane, ethane, and benzene have zero dipole moments. Because of the symmetrical structures of these molecules, the individual bond polarities and lone-pair contributions exactly cancel.
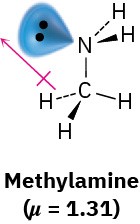
 Worked Example 2.1Predicting the Direction of a Dipole MomentMake a three-dimensional drawing of methylamine, CH3NH2, and show the direction of its dipole moment (μ = 1.31).StrategyLook for any lone-pair electrons, and identify any atom with an electronegativity substantially different from that of carbon. (Usually, this means O, N, F, Cl, or Br.) Electron density will be displaced in the general direction of the electronegative atoms and the lone pairs.SolutionMethylamine contains an electronegative nitrogen atom with a lone pair of electrons. The dipole moment thus points generally from –CH3 toward the lone pair.
Worked Example 2.1Predicting the Direction of a Dipole MomentMake a three-dimensional drawing of methylamine, CH3NH2, and show the direction of its dipole moment (μ = 1.31).StrategyLook for any lone-pair electrons, and identify any atom with an electronegativity substantially different from that of carbon. (Usually, this means O, N, F, Cl, or Br.) Electron density will be displaced in the general direction of the electronegative atoms and the lone pairs.SolutionMethylamine contains an electronegative nitrogen atom with a lone pair of electrons. The dipole moment thus points generally from –CH3 toward the lone pair.
Problem 2-5
Ethylene glycol, HOCH2CH2OH, may look nonpolar when drawn, but an internal hydrogen bond between the two –OH groups results in a dipole moment. Explain.
Problem 2-6
Make three-dimensional drawings of the following molecules, and predict whether each has a dipole moment. If you expect a dipole moment, show its direction.
(a) H2C=CH2
(b) CHCl3
(c) CH2Cl2
(d) H2C=CCl2