15.6 Polycyclic Aromatic Compounds
The Hückel rule is strictly applicable only to monocyclic compounds, but the general concept of aromaticity can be extended to include polycyclic aromatic compounds.
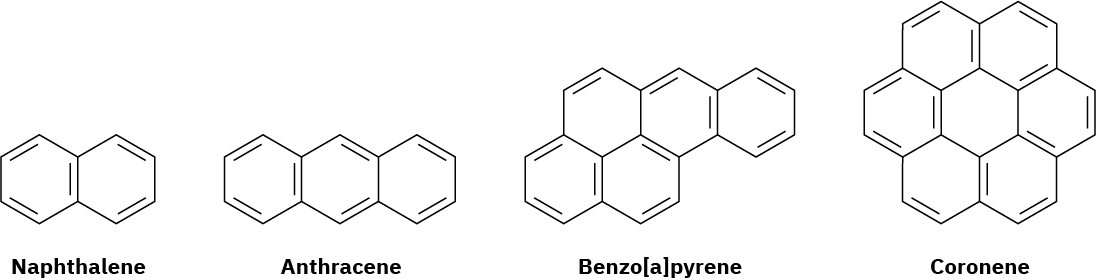
Naphthalene, with two benzene-like rings fused together; anthracene, with three rings; benzo[a]pyrene, with five rings; and coronene, with six rings, are all well-known aromatic hydrocarbons. Benzo[a]pyrene is particularly interesting because it is one of the cancer- causing substances found in tobacco smoke.
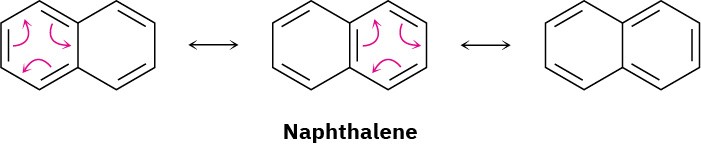
All polycyclic aromatic hydrocarbons can be represented by a number of different resonance forms. Naphthalene, for instance, has three.
Naphthalene and other polycyclic aromatic hydrocarbons show many of the chemical properties associated with aromaticity. Thus, measurement of its heat of hydrogenation shows an aromatic stabilization energy of approximately 250 kJ/mol (60 kcal/mol).
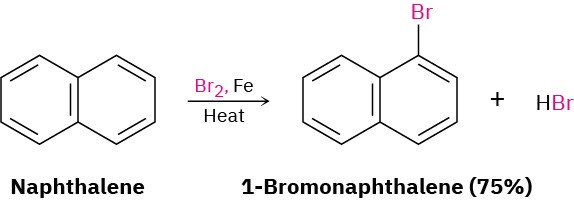
Furthermore, naphthalene reacts slowly with electrophiles such as Br2 to give substitution products rather than double-bond addition products.
The aromaticity of naphthalene is explained by the orbital picture in Figure 15.11. Naphthalene has a cyclic, conjugated π electron system, with p orbital overlap both along the ten-carbon periphery of the molecule and across the central bond. Because ten π electrons is a Hückel number, there is π electron delocalization and consequent aromaticity in naphthalene.
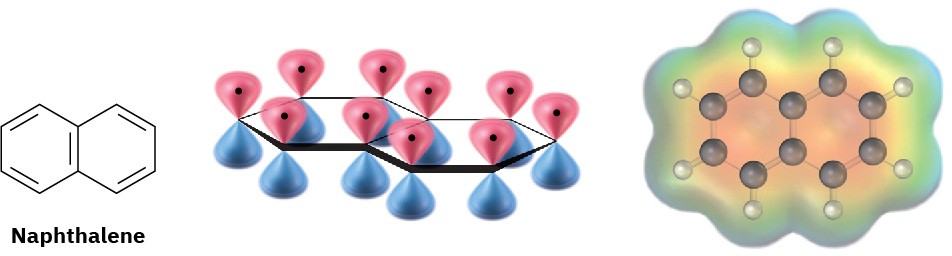
Figure 15.11An orbital picture and electrostatic potential map of naphthalene, showing that the ten π electrons are fully delocalized throughout both rings.
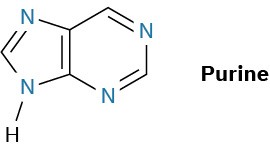
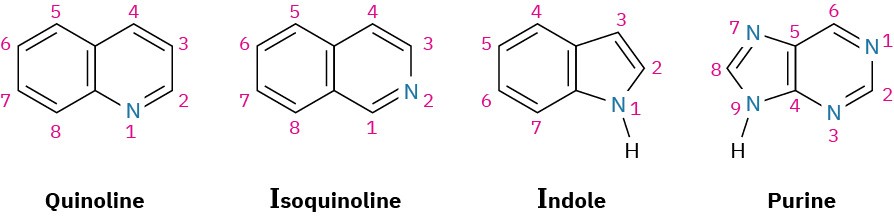
Just as there are heterocyclic analogs of benzene, there are also many heterocyclic analogs of naphthalene. Among the most common are quinoline, isoquinoline, indole, and purine. Quinoline, isoquinoline, and purine all contain pyridine-like nitrogens that are part of a double bond and contribute one electron to the aromatic π system. Indole and purine both contain pyrrole-like nitrogens that contribute two π electrons.
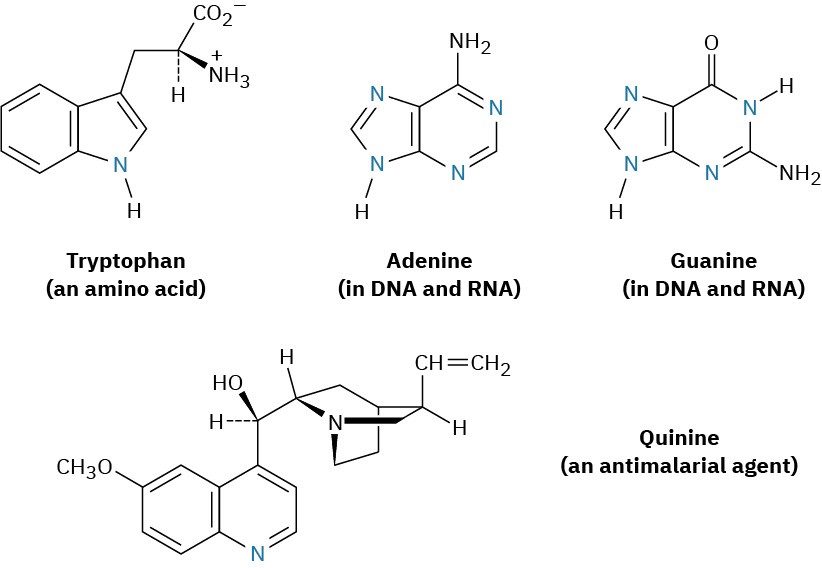
Among the many biological molecules that contain polycyclic aromatic rings, the amino acid tryptophan contains an indole ring and the antimalarial drug quinine contains a quinoline ring. Adenine and guanine, two of the five heterocyclic amine bases found in nucleic acids, have rings based on purine.
Problem 15-11
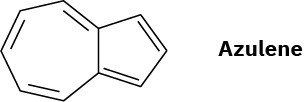
Azulene, a beautiful blue hydrocarbon, is an isomer of naphthalene. Is azulene aromatic? Draw a second resonance form of azulene in addition to that shown.
Problem 15-12
How many electrons does each of the four nitrogen atoms in purine contribute to the aromatic π system?