2.9 Predicting Acid–Base Reactions from pKa Values
Compilations of pKa values like those in Table 2.3 and Appendix B are useful for predicting whether a given acid–base reaction will take place, because H+ will always go from the stronger acid to the stronger base. That is, an acid will donate a proton to the conjugate base of a weaker acid, and the conjugate base of a weaker acid will remove a proton from a stronger acid. Since water (pKa = 15.74) is a weaker acid than acetic acid (pKa = 4.76), for example, hydroxide ion holds a proton more tightly than acetate ion does. Hydroxide ion will therefore react to a large extent with acetic acid, CH3CO2H, to yield acetate ion and H2O.
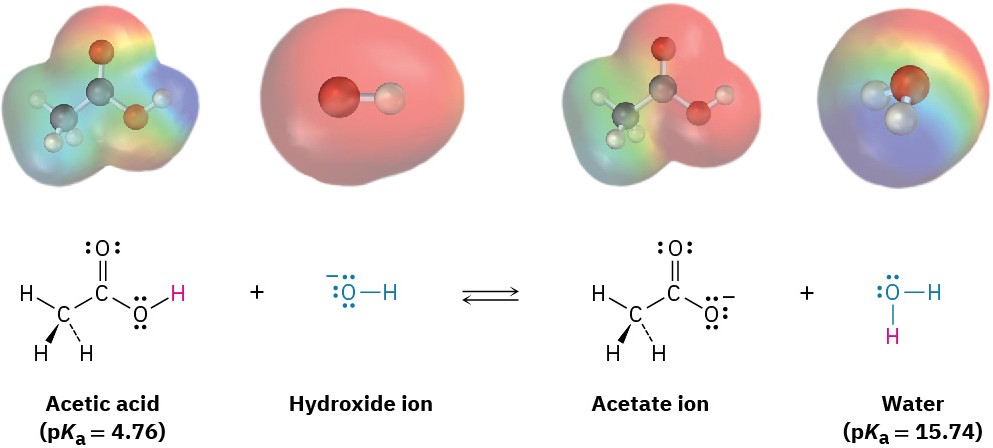
Another way to predict acid–base reactivity is to remember that the product conjugate acid in an acid–base reaction must be weaker and less reactive than the starting acid and the product conjugate base must be weaker and less reactive than the starting base. In the reaction of acetic acid with hydroxide ion, for example, the product conjugate acid (H2O) is weaker than the starting acid (CH3CO2H), and the product conjugate base (CH3CO2–) is weaker than the starting base (OH–).
 Worked Example 2.4Predicting Acid Strengths from pKa ValuesWater has pKa = 15.74, and acetylene has pKa = 25. Which is the stronger acid? Does hydroxide ion react to a significant extent with acetylene?
Worked Example 2.4Predicting Acid Strengths from pKa ValuesWater has pKa = 15.74, and acetylene has pKa = 25. Which is the stronger acid? Does hydroxide ion react to a significant extent with acetylene?
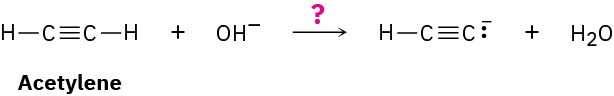
StrategyIn comparing two acids, the one with the lower pKa is stronger. Thus, water is a stronger acid than acetylene and gives up H+ more easily.SolutionBecause water is a stronger acid and gives up H+ more easily than acetylene, the HO– ion must have less affinity for H+ than the HC≡C:− ion. In other words, the anion of acetylene is a stronger base than hydroxide ion, and the reaction will not proceed significantly as written.
Worked Example 2.5Calculating Ka from pKaAccording to the data in Table 2.3, acetic acid has pKa = 4.76. What is its Ka?StrategySince pKa is the negative logarithm of Ka, it’s necessary to use a calculator with an ANTILOG or INV LOG function. Enter the value of the pKa (4.76), change the sign (–4.76), and then find the antilog (1.74 × 10–5).SolutionKa = 1.74 × 10–5.
Problem 2-14
Will either of the following reactions take place to a significant extent as written, according to the data in Table 2.3?
(a) 
(b) 
Problem 2-15
Ammonia, NH3, has pKa ≈ 36, and acetone has pKa ≈ 19. Will the following reaction take place to a significant extent?
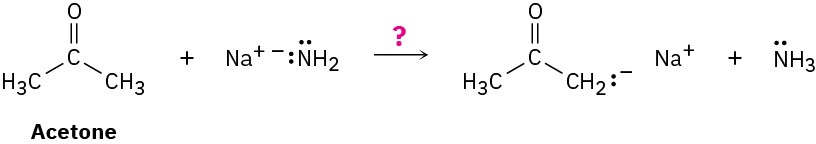
Problem 2-16
What is the Ka of HCN if its pKa = 9.31?

