19.2 Preparing Aldehydes and Ketones
Preparing Aldehydes
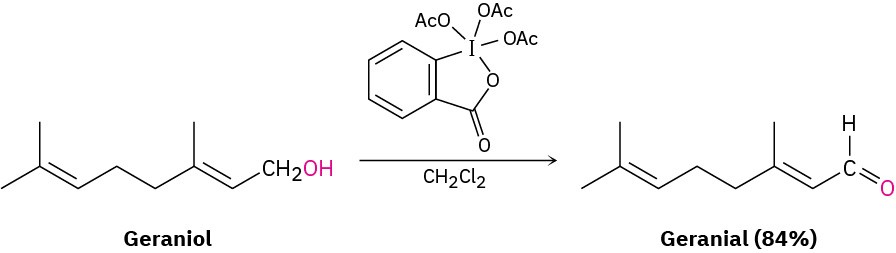
Perhaps the best method of aldehyde synthesis is by oxidation of a primary alcohol, as we saw in Section 17.7. The reaction is usually carried out using the Dess–Martin periodinane reagent in dichloromethane solvent at room temperature:
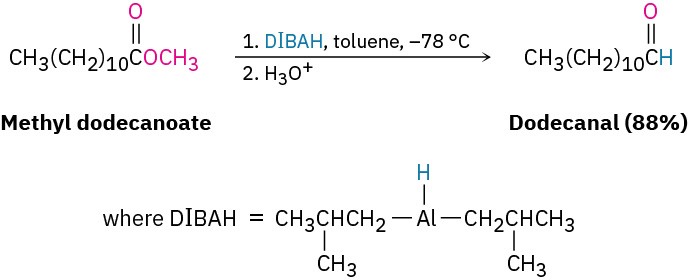
Another method of aldehyde synthesis is one that we’ll mention here just briefly and then return to in Section 21.6. Certain carboxylic acid derivatives can be partially reduced to yield aldehydes. The partial reduction of an ester by diisobutylaluminum hydride (DIBAH, or DIBAL-H), for instance, is an important laboratory-scale method of aldehyde synthesis, and mechanistically related processes also occur in biological pathways. The reaction is normally carried out at –78 °C (dry-ice temperature) in toluene solution.
Problem 19-3
How would you prepare pentanal from the following starting materials? (a)
CH3CH2CH2CH2CH2OH
(b) CH3CH2CH2CH2CH=CH2
(c) CH3CH2CH2CH2CO2CH3
(d)
CH3CH2CH2CH=CH2
Preparing Ketones
For the most part, methods of ketone synthesis are similar to those for aldehydes. Secondary alcohols are oxidized by a variety of reagents to give ketones (Section 17.7). The choice of oxidant depends on such factors as reaction scale, cost, and acid or base sensitivity of the alcohol. The Dess–Martin periodinane is a common choice although chromic acid (H2CrO4) was frequently used in older work.
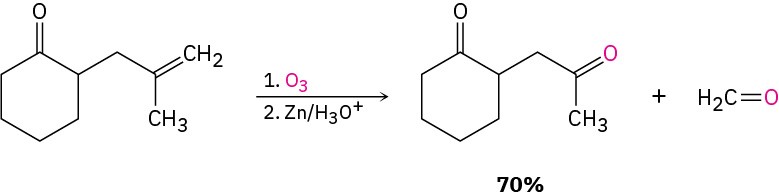
Other methods include the ozonolysis of alkenes in which one of the unsaturated carbon atoms is disubstituted (Section 8.8) and Friedel–Crafts acylation of an aromatic ring with an acid chloride in the presence of AlCl3 catalyst (Section 16.3).
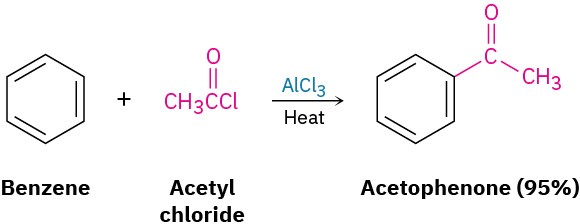
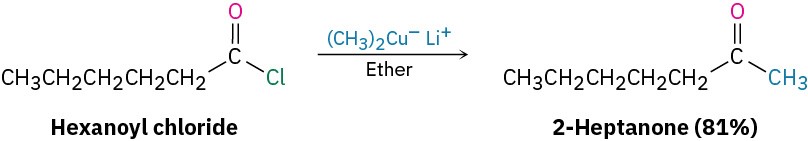
In addition to those methods already discussed, ketones can also be prepared from certain carboxylic acid derivatives, just as aldehydes can. Among the most useful reactions of this sort is that between an acid chloride and a lithium diorganocopper reagent, as we saw in Section 10.7. We’ll discuss this reaction in more detail in Section 21.4.
Problem 19-4
How would you carry out the following reactions? More than one step may be needed. (a)
3-Hexyne → 3-Hexanone (b)
Benzene → m-Bromoacetophenone (c)
Bromobenzene → Acetophenone (d)
1-Methylcyclohexene → 2-Methylcyclohexanone

