26.9 Protein Structure
Proteins are usually classified as either fibrous or globular, according to their three- dimensional shape. Fibrous proteins, such as the collagen in tendons and connective tissue and the myosin in muscle tissue, consist of polypeptide chains arranged side by side in long filaments. Because these proteins are tough and insoluble in water, they are used in nature for structural materials. Globular proteins, by contrast, are usually coiled into compact, roughly spherical shapes. These proteins are generally soluble in water and are mobile within cells. Most of the more than 3000 or so enzymes that have been characterized are globular proteins, including the 1300 different enzymes in the human body.
Proteins are so large that the word structure takes on a broader meaning than with simpler organic compounds. In fact, chemists speak of four different levels of structure when describing proteins.
- The primary structure of a protein is simply the amino acid sequence.
- The secondary structure of a protein describes how segments of the peptide backbone orient into a regular pattern.
- The tertiary structure describes how the entire protein molecule coils into an overall three-dimensional shape.
- The quaternary structure describes how different protein molecules come together to yield large aggregate structures.
Primary structure is determined, as we’ve seen, by sequencing the protein. Secondary, tertiary, and quaternary structures are determined either by NMR or by X-ray crystallography (Chapter 12 Chemistry Matters).
The most common secondary structures are the α helix and the β-pleated sheet. An α helix is a right-handed coil of the protein backbone, much like the coil of a spiral staircase (Figure 26.6a). Each turn of the helix contains 3.6 amino acid residues, with a distance between coils of 540 pm, or 5.4 Å. The structure is stabilized by hydrogen bonds between amide N–H groups and C═O groups four residues away, with an N–HO distance of 2.8 Å.
The α helix is an extremely common secondary structure, and nearly all globular proteins contain many helical segments. Myoglobin, a small globular protein containing 153 amino acid residues in a single chain, is one example (Figure 26.6b).
A β-pleated sheet differs from an α helix in that the peptide chain is fully extended rather than coiled and the hydrogen bonds occur between residues in adjacent chains (Figure 26.7a). The neighboring chains can run either in the same direction (parallel) or in opposite directions (antiparallel), although the antiparallel arrangement is more common and somewhat more energetically favorable. Concanavalin A, for instance, consists of two identical chains of 237 residues, with extensive regions of antiparallel β sheets (Figure 26.7b).
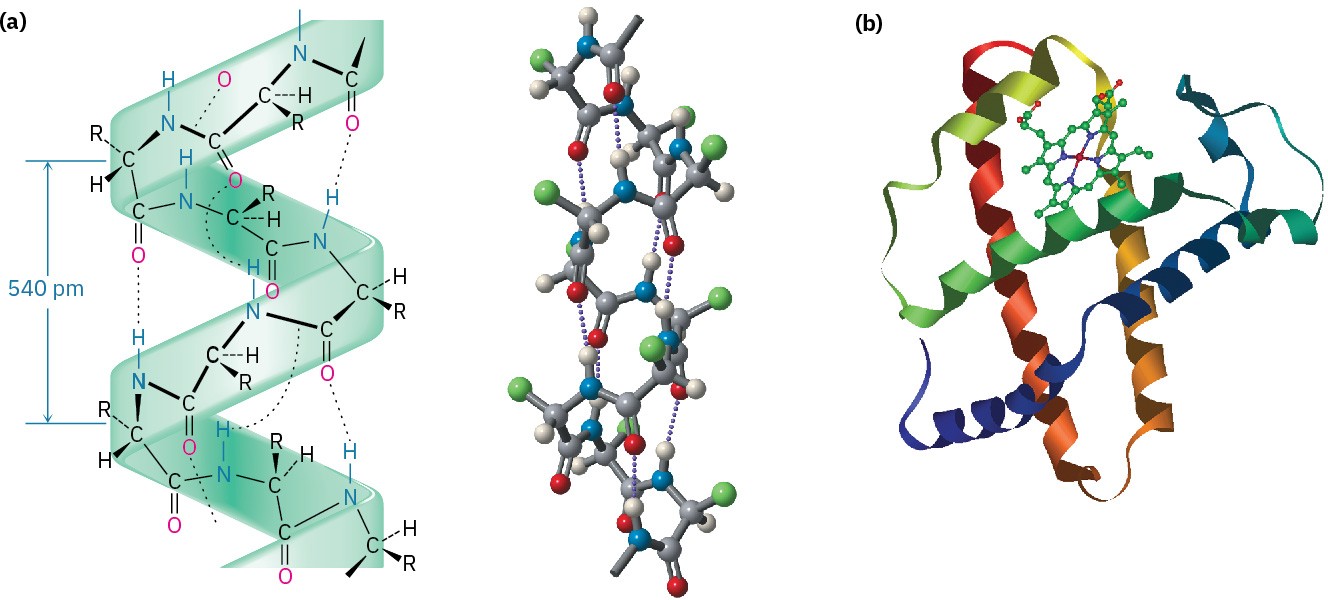
Figure 26.6 (a) The α-helical secondary structure of proteins is stabilized by hydrogen bonds between the N–H group of one residue and the C═O group four residues away. (b) The structure of myoglobin, a globular protein with extensive helical regions that are shown as coiled ribbons in this representation.
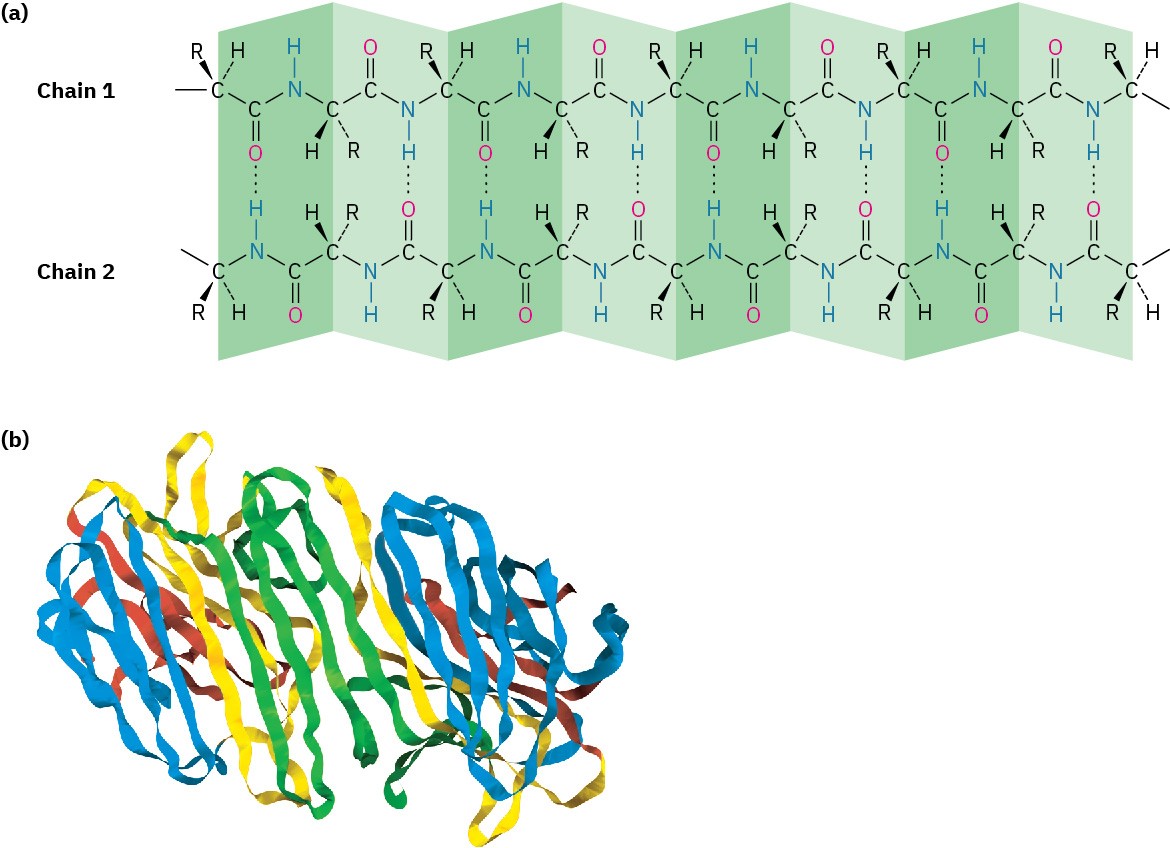
Figure 26.7 (a) The β-pleated sheet secondary structure of proteins is stabilized by hydrogen bonds between parallel or antiparallel chains. (b) The structure of concanavalin A, a protein with extensive regions of antiparallel β sheets, shown as flat ribbons.
What about tertiary structure? Why does a protein adopt the shape it does? The forces that determine the tertiary structure of a protein are the same forces that act on all molecules, regardless of size, to provide maximum stability. Particularly important are the hydrophilic (water-loving; Section 2.12) interactions of the polar side chains on acidic or basic amino acids and the hydrophobic (water-fearing) interactions of nonpolar side chains. These acidic or basic amino acids with charged side chains tend to congregate on the exterior of the protein, where they can be solvated by water. Amino acids with neutral, nonpolar side chains tend to congregate on the hydrocarbon-like interior of a protein molecule, away from the aqueous medium.
Also important for stabilizing a protein’s tertiary structure are the formation of disulfide bridges between cysteine residues, the formation of hydrogen bonds between nearby amino acid residues, and the presence of ionic attractions, called salt bridges, between positively and negatively charged sites on various amino acid side chains within the protein.
Because the tertiary structure of a globular protein is delicately maintained by weak intramolecular attractions, a modest change in temperature or pH is often enough to disrupt that structure and cause the protein to become denatured. Denaturation occurs under such mild conditions that the primary structure remains intact, but the tertiary structure unfolds from a specific globular shape to a randomly looped chain (Figure 26.8).
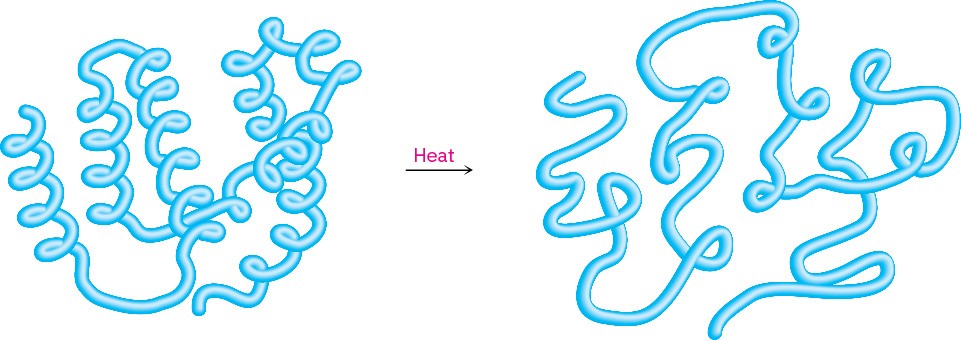
Figure 26.8 A representation of protein denaturation. A globular protein loses its specific three-dimensional shape and becomes randomly looped.
Denaturation is accompanied by changes in both physical and biological properties. Solubility is drastically decreased, as occurs when egg white is cooked and the albumins unfold and coagulate. Most enzymes lose all catalytic activity when denatured, since a precisely defined tertiary structure is required for their action. Although most denaturation is irreversible, some cases are known where spontaneous renaturation of an unfolded protein to its stable tertiary structure occurs, accompanied by a full recovery of biological activity.

