6.6 Radical Reactions
Radical reactions are much less common than polar reactions but are nevertheless important in some industrial processes and biological pathways. We’ll look at them in more detail in Sections 10.2 and 10.3 but will briefly see how they occur at this point.
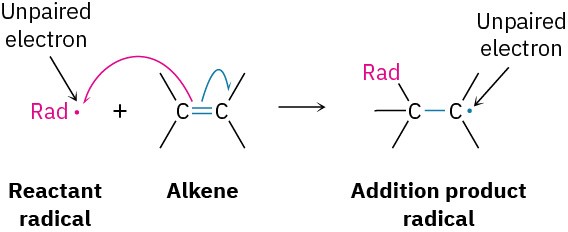
A radical is highly reactive because it contains an atom with an odd number of electrons (usually seven) in its valence shell, rather than a noble-gas octet. The radical can achieve a valence-shell octet in several ways however. For instance, it might abstract an atom and one bonding electron from another reactant, leaving behind a new radical. The net result is a radical substitution reaction.
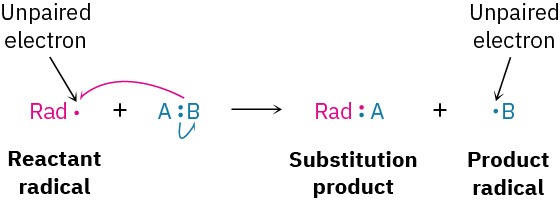
Alternatively, a reactant radical might add to a double bond, taking one electron from the double bond and yielding a new radical. The net result is a radical addition reaction.
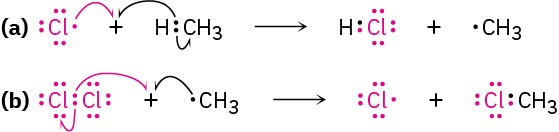
An example of an industrially useful radical reaction is the reaction of chlorine with methane to yield chloromethane, which is used to manufacture the solvents dichloromethane (CH2Cl2) and chloroform (CHCl3). The process begins with irradiation of Cl2 with ultraviolet light to break the relatively weak Cl−Cl bond of Cl2 and produce chlorine radicals (·Cl).
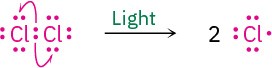
Chlorine radicals then react with methane by abstracting a hydrogen atom to give HCl and a methyl radical (·CH3) that reacts further with Cl2 to give chloromethane plus a new chlorine radical that cycles back and repeats the first step. Thus, once the sequence has started, it becomes a self-sustaining cycle of repeating steps (a) and (b), making the overall process a chain reaction.
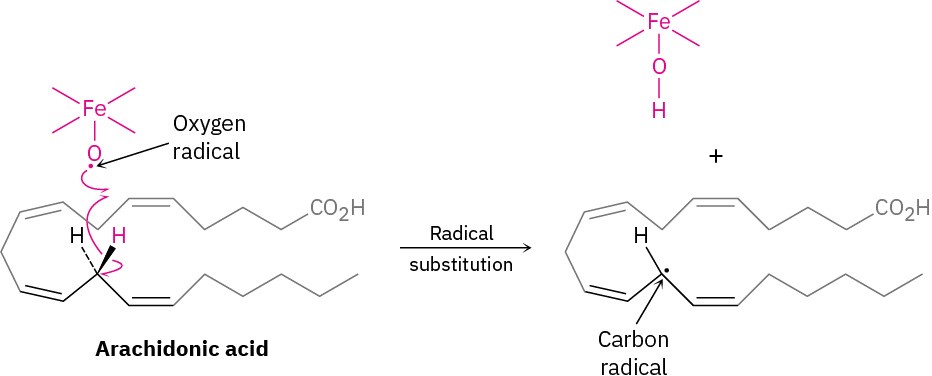
As a biological example of a radical reaction, look at the synthesis of prostaglandins, a large class of molecules found in virtually all body tissues and fluids. A number of pharmaceuticals are based on or derived from prostaglandins, including medicines that induce labor during childbirth, reduce intraocular pressure in glaucoma, control bronchial asthma, and help treat congenital heart defects.
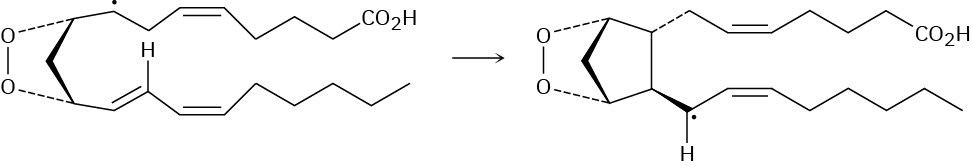
Prostaglandin biosynthesis is initiated by the abstraction of a hydrogen atom from arachidonic acid by an iron–oxygen radical, thereby generating a carbon radical in a substitution reaction. Don’t be intimidated by the size of the molecules; focus on the changes that occur in each step. (To help you do that, the unchanged part of the molecule is “ghosted,” with only the reactive part clearly visible.)
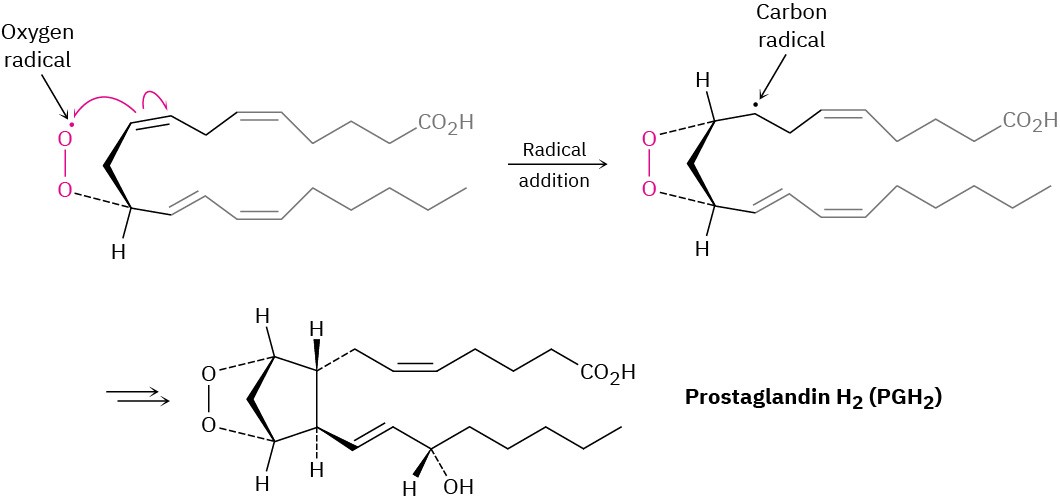
Following the initial abstraction of a hydrogen atom, the carbon radical then reacts with O2 to give an oxygen radical, which reacts with a C═C bond within the same molecule in an addition reaction. Several further transformations ultimately yield prostaglandin H2.
Problem 6-8
Radical chlorination of alkanes is not generally useful because mixtures of products often result when more than one kind of C−H bond is present in the substrate. Draw and name all monochloro substitution products C6H13Cl you might obtain by reaction of 2- methylpentane with Cl2.
Problem 6-9
Using curved fishhook arrows, propose a mechanism for the formation of the cyclopentane ring of prostaglandin H2.