22.6 Reactivity of Enolate Ions
Enolate ions are more useful than enols for two reasons. First, pure enols can’t normally be isolated but are instead generated only as short-lived intermediates in low concentration. By contrast, stable solutions of pure enolate ions are easily prepared from most carbonyl compounds by reaction with a strong base. Second, enolate ions are more reactive than enols and undergo many reactions that enols don’t. Whereas enols are neutral, enolate ions are negatively charged, making them much better nucleophiles.
Because they are resonance hybrids of two nonequivalent forms, enolate ions can be looked at either as vinylic alkoxides (C═C−O!) or as α-keto carbanions (!C−C═O). Thus, enolate ions can react with electrophiles either on oxygen or on carbon. Reaction on oxygen yields an enol derivative, while reaction on carbon yields an α-substituted carbonyl compound (Figure 22.6). Both kinds of reactivity are known, but reaction on carbon is more common.
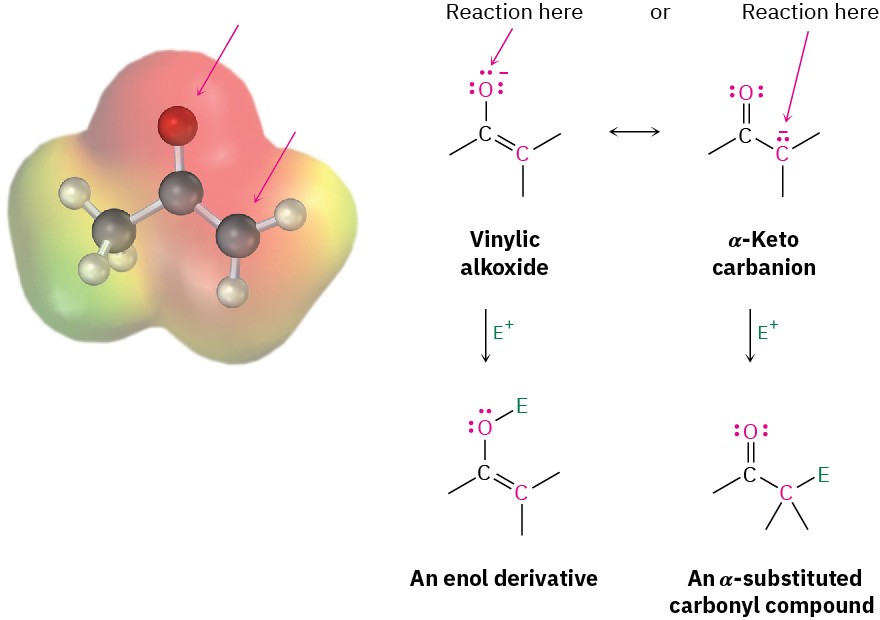
Figure 22.6 The electrostatic potential map of acetone enolate ion shows how the negative charge is delocalized over both the oxygen and the α carbon. As a result, two modes of reaction of an enolate ion with an electrophile E+ are possible. Reaction on carbon to yield an α-substituted carbonyl product is more common.
As an example of enolate ion reactivity, aldehydes and ketones undergo base-promoted α halogenation. Even relatively weak bases such as hydroxide ion are effective for halogenation because it’s not necessary to convert the ketone completely into its enolate ion. As soon as a small amount of enolate is generated, halogen reacts with it immediately, removing it from the reaction and driving the equilibrium toward further enolate ion formation.
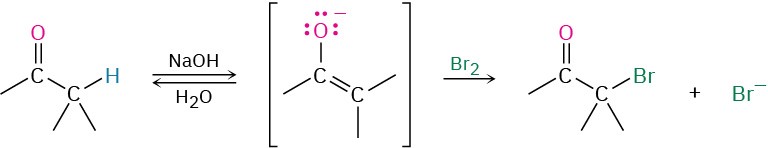
Base-promoted halogenation of aldehydes and ketones is seldom used in practice because it’s difficult to stop the reaction at the monosubstituted product. An α-halogenated ketone is generally more acidic than the starting, unsubstituted ketone because of the electron- withdrawing inductive effect of the halogen atom. Thus, the monohalogenated products are themselves rapidly turned into enolate ions and further halogenated.
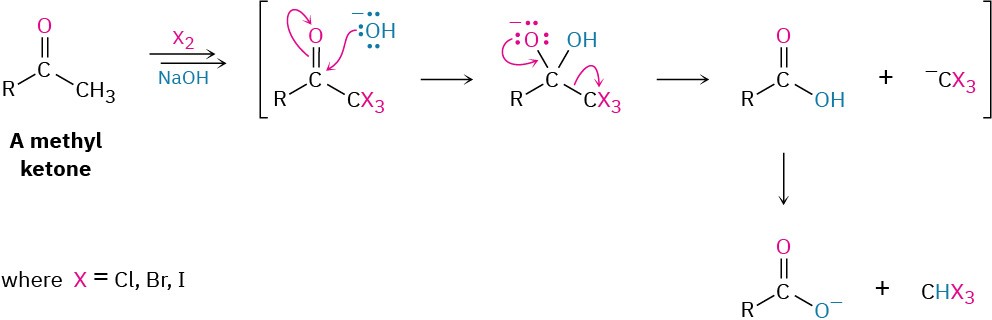
If excess base and halogen are used, a methyl ketone is triply halogenated and then cleaved by base in the haloform reaction. The products are a carboxylic acid plus a so-called haloform (chloroform, CHCl3; bromoform, CHBr3; or iodoform, CHI3). Note that the second step of the reaction is a nucleophilic acyl substitution of –CX3 by –OH. That is, a halogen- stabilized carbanion acts as a leaving group.
Problem 22-9
Why do you suppose ketone halogenations in acidic media are referred to as being acid- catalyzed, whereas halogenations in basic media are base-promoted? In other words, why is a full equivalent of base required for halogenation?

