17.11 Spectroscopy of Alcohols and Phenols
Infrared Spectroscopy
Alcohols have a strong C–O stretching absorption near 1050 cm–1 and a characteristic O–H stretching absorption at 3300 to 3600 cm–1. The exact position of the O–H stretch depends on the extent of hydrogen-bonding in the molecule. Unassociated alcohols show a fairly sharp absorption near 3600 cm–1, whereas hydrogen-bonded alcohols show a broader absorption in the 3300 to 3400 cm–1 range. The hydrogen-bonded hydroxyl absorption appears at 3350 cm–1 in the IR spectrum of cyclohexanol (Figure 17.12).
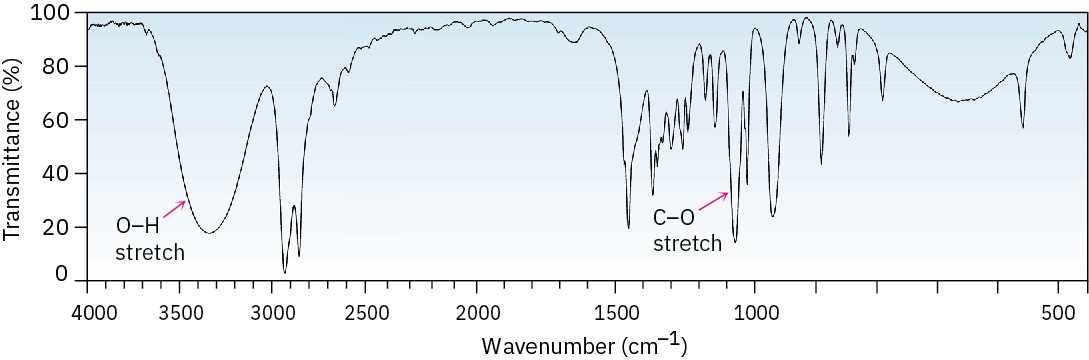
Figure 17.12 IR spectrum of cyclohexanol. Characteristic O–H and C–O stretching absorptions are indicated.
Phenols also show a characteristic broad IR absorption at 3500 cm–1 due to the –OH group, as well as the usual 1500 and 1600 cm–1 aromatic bands (Figure 17.13). In phenol itself, monosubstituted aromatic-ring peaks are visible at 690 and 760 cm–1.
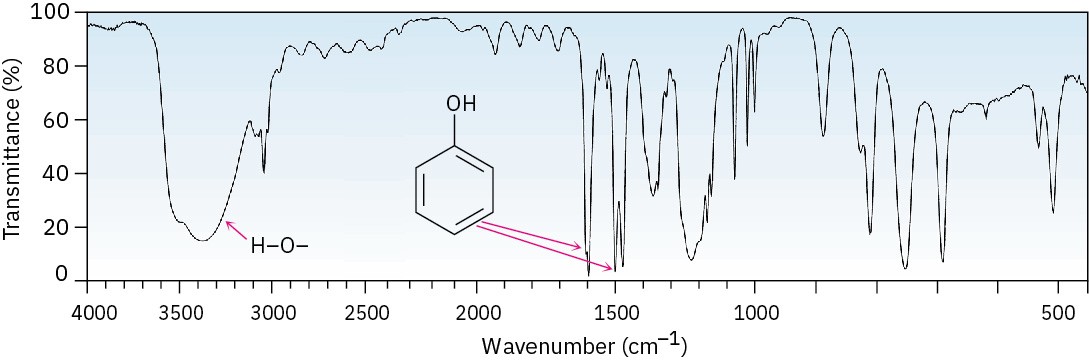
Figure 17.13 IR spectrum of phenol. Problem 17-18
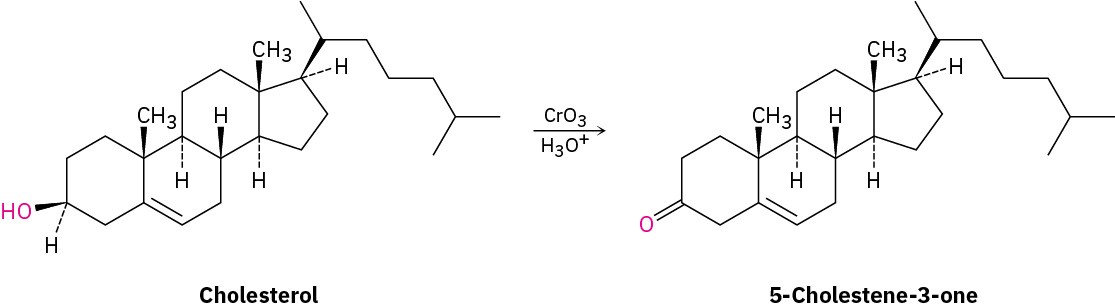
Assume that you need to prepare 5-cholesten-3-one from cholesterol. How could you use IR spectroscopy to tell whether the reaction was successful? What differences would you look for in the IR spectra of starting material and product?
Nuclear Magnetic Resonance Spectroscopy
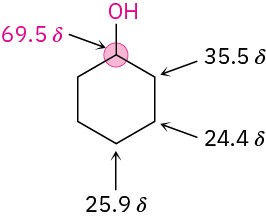
Carbon atoms bonded to electron-withdrawing –OH groups are deshielded and absorb at a lower field in the 13C NMR spectrum than do typical alkane carbons. Most alcohol carbon absorptions fall in the range 50 to 80 δ, as shown in the following drawing for cyclohexanol:
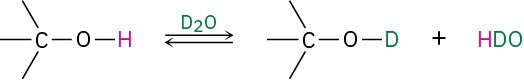
Alcohols also show characteristic absorptions in the 1H NMR spectrum. Hydrogens on the oxygen-bearing carbon atom are deshielded by the electron-withdrawing effect of the nearby oxygen, and their absorptions occur in the range 3.4 to 4.5 δ. Spin–spin splitting, however, is not usually observed between the O–H proton of an alcohol and the neighboring protons on carbon. Most samples contain small amounts of acidic impurities, which catalyze an exchange of the O–H proton on a timescale so rapid that the effect of spin–spin splitting is removed. It’s often possible to take advantage of this rapid proton exchange to identify the position of the O–H absorption. If a small amount of deuterated water, D2O, is added to an NMR sample tube, the O–H proton is rapidly exchanged for deuterium and the hydroxyl absorption disappears from the spectrum.
Typical spin–spin splitting is observed between protons on the oxygen-bearing carbon and other neighbors. For example, the signal of the two –CH2O– protons in 1-propanol is split into a triplet by coupling with the neighboring –CH2– protons (Figure 17.14).
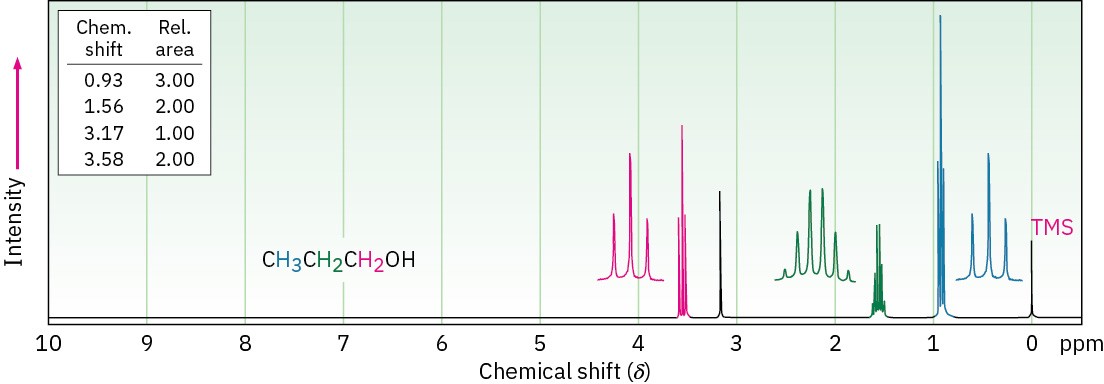
Figure 17.14 1H NMR spectrum of 1-propanol. The protons on the oxygen-bearing carbon are split into a triplet at 3.58 δ.
Phenols, like all aromatic compounds, show 1H NMR absorptions near 7 to 8 δ, the expected position for aromatic-ring protons (Section 15.7). In addition, phenol O–H protons absorb at 3 to 8 δ. In neither case are these absorptions uniquely diagnostic for phenols, since other kinds of protons absorb in the same range.
Problem 17-19
When the 1H NMR spectrum of an alcohol is run in dimethyl sulfoxide (DMSO) solvent rather than in chloroform, exchange of the O–H proton is slow and spin–spin splitting is seen between the O–H proton and C–H protons on the adjacent carbon. What spin multiplicities would you expect for the hydroxyl protons in the following alcohols?
(a)
2-Methyl-2-propanol (b)
Cyclohexanol (c)
Ethanol (d)
2-Propanol (e) Cholesterol (f)
- Methylcyclohexanol
Mass Spectrometry
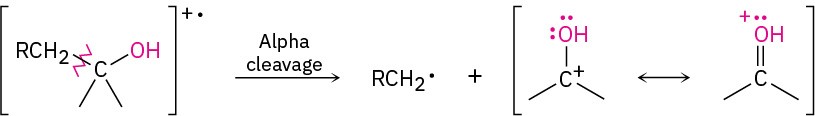
As noted in Section 12.3, alcohols undergo fragmentation in the mass spectrometer by two characteristic pathways, alpha cleavage and dehydration. In the alpha-cleavage pathway, a C–C bond nearest the hydroxyl group is broken, yielding a neutral radical plus a resonance- stabilized, oxygen-containing cation.
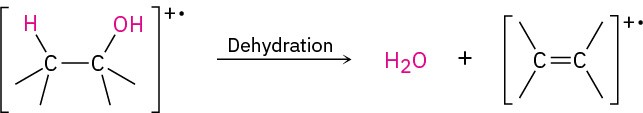
In the dehydration pathway, water is eliminated, yielding an alkene radical cation.
Both fragmentation modes are apparent in the mass spectrum of 1-butanol (Figure 17.15). The peak at m/z = 56 is due to loss of water from the molecular ion, and the peak at m/z = 31 is due to an alpha cleavage.
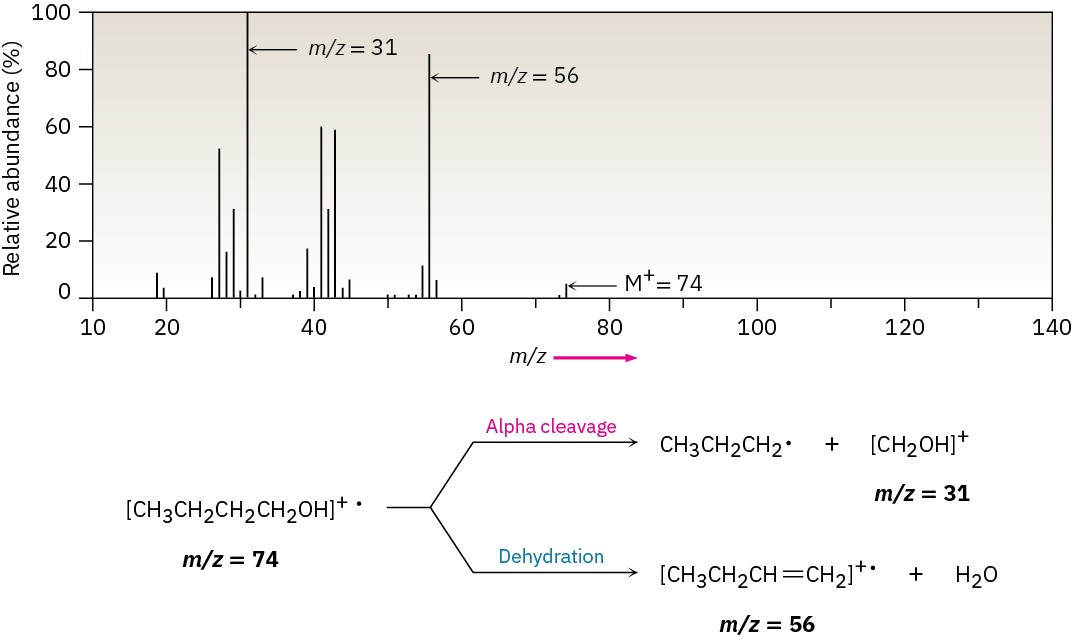
Figure 17.15 Mass spectrum of 1-butanol (M+ = 74). Dehydration gives a peak at m/z = 56, and fragmentation by alpha cleavage gives a peak at m/z = 31.
Additional Problems 17 • Additional Problems 17 • Additional Problems Visualizing Chemistry Problem 17-20
Give IUPAC names for the following compounds: (a)
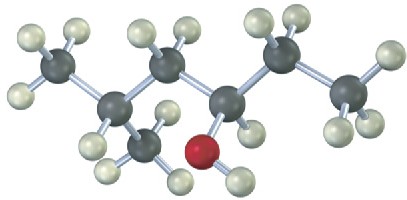
(b)
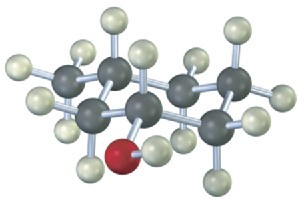
(c)
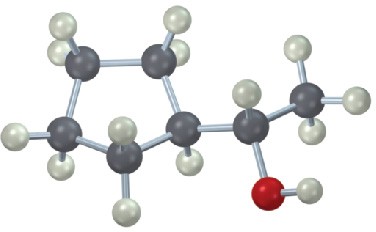
(d)
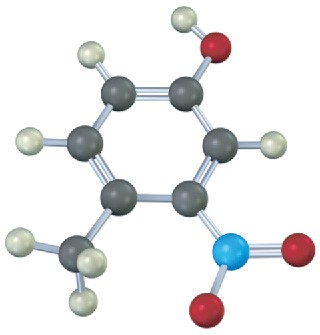
Problem 17-21
Draw the structure of the carbonyl compound(s) from which each of the following alcohols might have been prepared, and show the products you would obtain by treatment of each alcohol with (1) Na metal, (2) SOCl2, and (3) Dess–Martin periodinane.
(a)
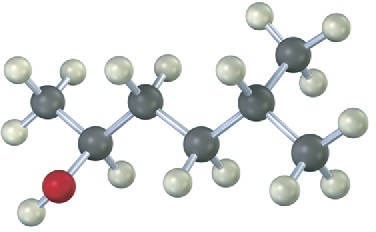
(b)
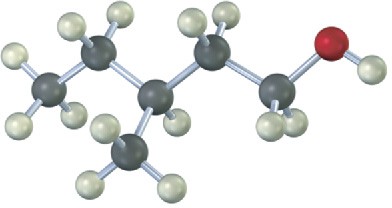
Problem 17-22
Predict the product from reaction of the following substance (reddish brown = Br) with:
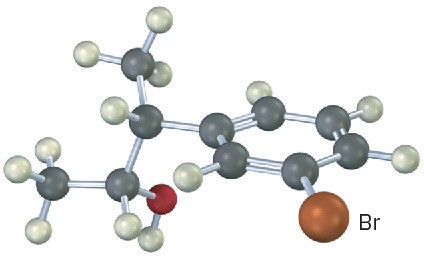
(a) PBr3
(b)
Aqueous H2SO4 (c)
SOCl2
(d)
Dess–Martin periodinane (e)
Br2, FeBr3 Problem 17-23
Predict the product from reaction of the following substance with:
(a)
NaBH4; then H3O+ (b)
LiAlH4; then H3O+ (c)
2 CH3CH2MgBr; then H3O+ Problem 17-24
Name and assign R or S stereochemistry to the product(s) you would obtain by reaction of the following substance with ethylmagnesium bromide. Is the product chiral? Is it optically active? Explain.
Mechanism Problems
Problem 17-25
Evidence for the intermediate carbocations in the acid-catalyzed dehydration of alcohols comes from the observation that rearrangements sometimes occur. Propose a mechanism to account for the formation of 2,3-dimethyl-2-butene from 3,3-dimethyl-2-butanol.
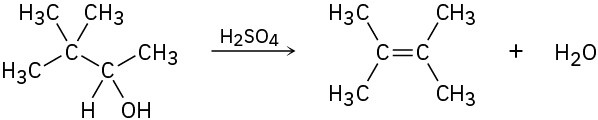
Problem 17-26
Acid-catalyzed dehydration of 2,2-dimethylcyclohexanol yields a mixture of 1,2- dimethylcyclohexene and isopropylidenecyclopentane. Propose a mechanism to account for the formation of both products.

Problem 17-27
Epoxides react with Grignard reagents to yield alcohols. Propose a mechanism.
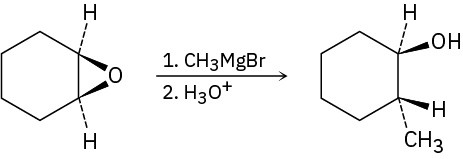
Problem 17-28
Treatment of the following epoxide with aqueous acid produces a carbocation intermediate that reacts with water to give a diol product. Show the structure of the carbocation, and propose a mechanism for the second step.
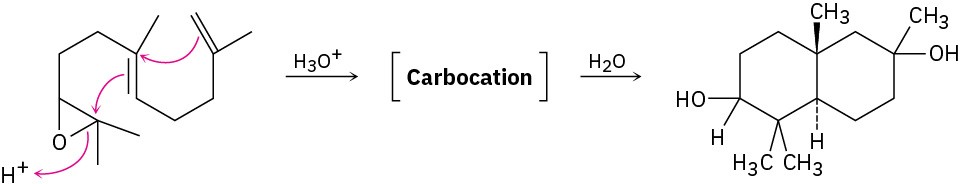
Problem 17-29
Reduction of 2-butanone with NaBH4 yields 2-butanol. Is the product chiral? Is it optically active? Explain.
Problem 17-30
The conversion of 3° alcohols into 3° alkyl halides under acidic conditions involves two cationic intermediates. For each reaction, draw the complete mechanism using curved arrows.
(a)
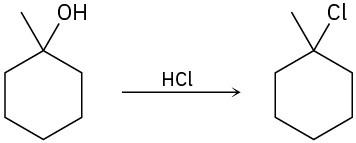
(b)
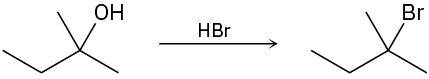
(c)
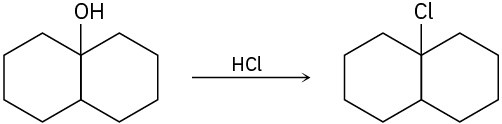
Problem 17-31
Identify the type of substitution mechanism (SN1, SN2) involved in the conversion of the following alcohols into the corresponding alkyl halide.
(a)
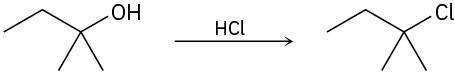
(b)
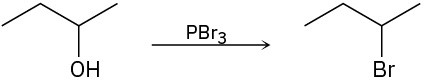
(c)
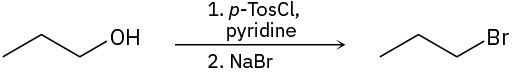
Problem 17-32
The conversion of 3° alcohols into alkenes under acidic conditions involves two cationic intermediates. For each reaction, draw the complete mechanism using curved arrows.
(a)
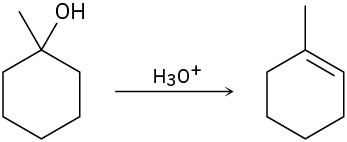
(b)
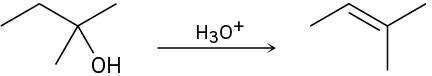
(c)
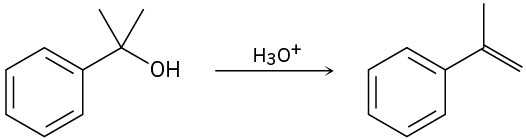
Problem 17-33
For each reaction, write the mechanism using curved arrows for the conversion of the alcohol into the corresponding alkene with POCl3. In each case, explain the regiochemistry of the elimination.
(a)
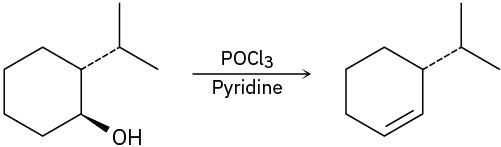
(b)
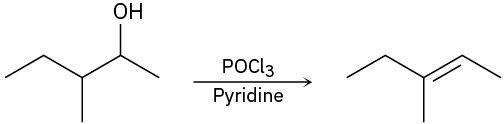
(c)
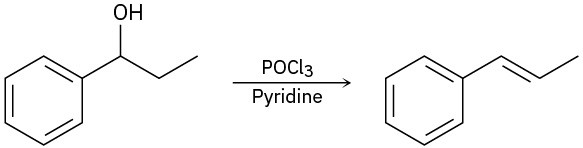
Problem 17-34
The trimethylsilyl (TMS) protecting group is one of several silicon protecting groups for alcohols. For each reaction, draw the mechanism for the protection of (R)-3-bromo-1- butanol with the following silyl chlorides, using triethylamine as the base:
(a)
tert-butyldimethylsilyl chloride (TBS-Cl) (b)
triisopropylsilyl chloride (TIPS-Cl) (c)
triethylsilyl chloride (TES-Cl) Problem 17-35
When the following alcohol is treated with POCl3 and pyridine, the expected elimination product is formed. However, when the same alcohol is treated with H2SO4, the elimination
product is 1,2-dimethylcyclopentene. Propose a mechanism for each pathway to account for these differences.
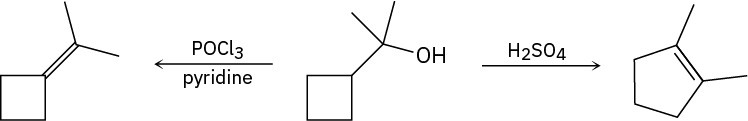
Problem 17-36
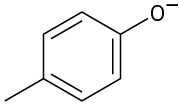
Phenols generally have lower pKa’s than alcohols because of resonance stabilization with the aromatic ring. Draw all of the resonance contributors for the following phenolate ions.
(a)
(b)
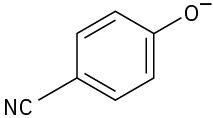
(c)
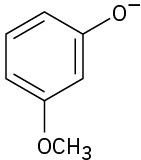
Naming Alcohols
Problem 17-37
Give IUPAC names for the following compounds: (a)
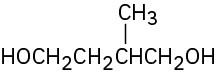
(b)
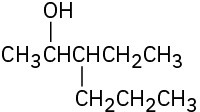
(c)
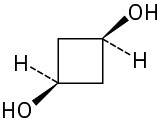
(d)
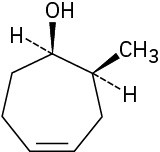
(e)
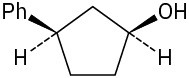
(f)
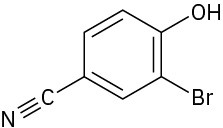
Problem 17-38
Draw and name the eight isomeric alcohols with formula C5H12O. Problem 17-39
Draw structures corresponding to the following IUPAC names: (a)
Trans-3-Chlorocycloheptanol (b)
- Ethyl-2-buten-1-ol (c)
o-(2-Hydroxyethyl)phenol (d)
- Methyl-1-phenyl-1-butanol Problem 17-40
Bombykol, the sex pheromone secreted by the female silkworm moth has the formula C16H28O and the systematic name (10E,12Z)-10,12-hexadecadien-1-ol. Draw bombykol, showing the correct geometry for the two double bonds.
Problem 17-41
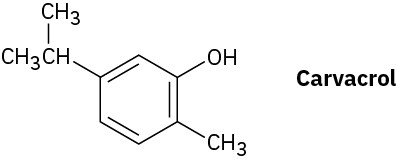
Carvacrol is a naturally occurring substance isolated from oregano, thyme, and marjoram. What is its IUPAC name?
Synthesizing Alcohols
Problem 17-42
What Grignard reagent and what carbonyl compound might you start with to prepare the following alcohols:
(a)
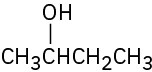
(b)
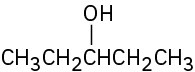
(c)
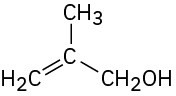
(d)
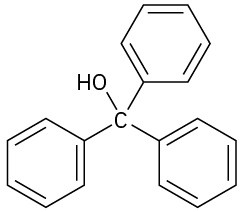
(e)
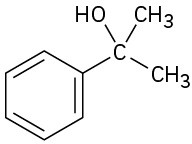
(f)
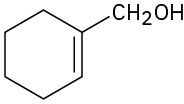
Problem 17-43
What carbonyl compounds would you reduce to prepare the following alcohols: List all possibilities.
(a)
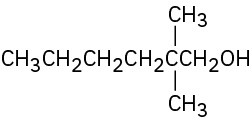
(b)
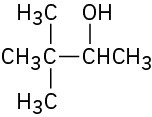
(c)
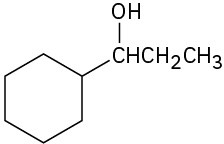
Problem 17-44
What carbonyl compounds might you start with to prepare the following compounds by Grignard reaction? List all possibilities.
(a)
2-Methyl-2-propanol (b)
1-Ethylcyclohexanol (c)
3-Phenyl-3-pentanol (d)
2-Phenyl-2-pentanol (e)
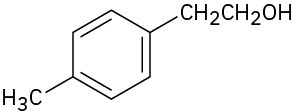
(f)
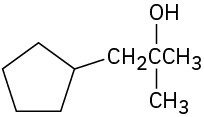
Problem 17-45
How would you synthesize the following alcohols, starting with benzene and other alcohols of six or fewer carbons as your only organic reagents?
(a)
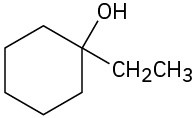
(b)
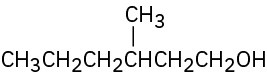
(c)
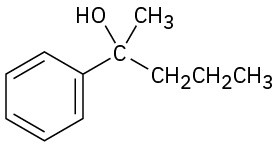
(d)
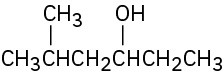
Reactions of Alcohols
Problem 17-46
What products would you obtain from reaction of 1-pentanol with the following reagents: (a)
PBr3
(b) SOCl2
(c)
Dess–Martin periodinane Problem 17-47
How would you prepare the following compounds from 2-phenylethanol: More than one step may be required.
(a)
Styrene (PhCH=CH2) (b)
Phenylacetaldehyde (PhCH2CHO) (c)
Phenylacetic acid (PhCH2CO2H) (d)
Benzoic acid (e) Ethylbenzene (f) Benzaldehyde (g)
1-Phenylethanol (h)
- Bromo-2-phenylethane Problem 17-48
How would you prepare the following compounds from 1-phenylethanol: More than one step may be required.
(a)
Acetophenone (PhCOCH3) (b)
Benzyl alcohol (c)
m-Bromobenzoic acid (d)
- Phenyl-2-propanol
Problem 17-49
How would you prepare the following substances from cyclopentanol: More than one step may be required.
(a) Cyclopentanone (b) Cyclopentene (c)
1-Methylcyclopentanol (d)
trans-2-Methylcyclopentanol Problem 17-50
What products would you expect to obtain from reaction of 1-methylcyclohexanol with the following reagents?
(a) HBr (b) NaH (c) H2SO4
Spectroscopy
Problem 17-51
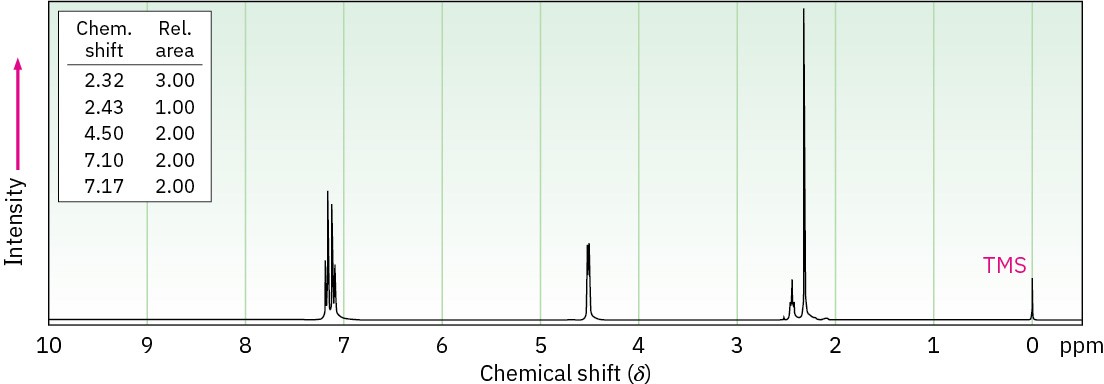
The following 1H NMR spectrum is that of an alcohol, C8H10O. Propose a structure.
Problem 17-52
Propose structures for alcohols that have the following 1H NMR spectra: (a)
C5H12O
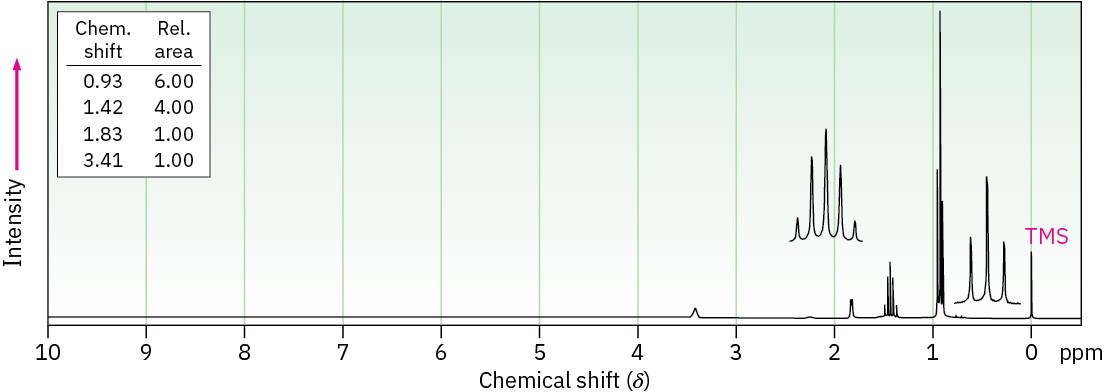
(b) C8H10O
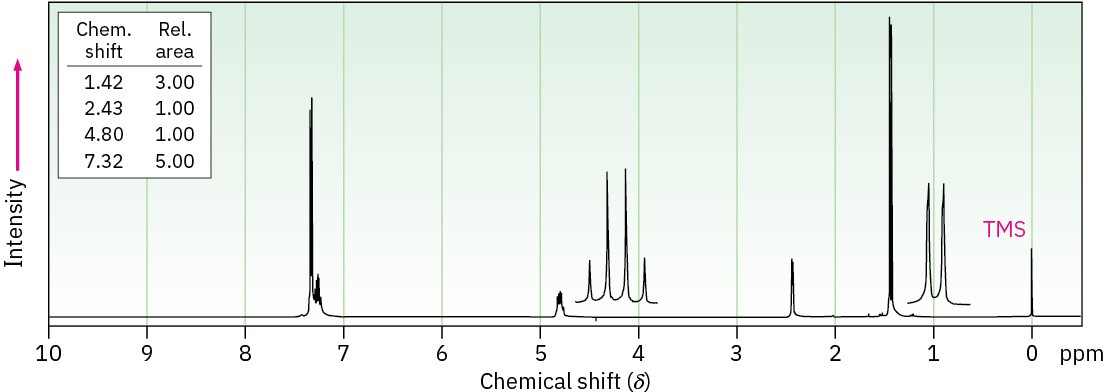
Problem 17-53
Propose a structure consistent with the following spectral data for a compound C8H18O2:
- IR: 3350 cm–1
- 1H NMR: 1.24 δ (12 H, singlet); 1.56 δ (4 H, singlet); 1.95 δ (2 H, singlet) Problem 17-54
The 1H NMR spectrum shown is that of 3-methyl-3-buten-1-ol. Assign all the observed resonance peaks to specific protons, and account for the splitting patterns.
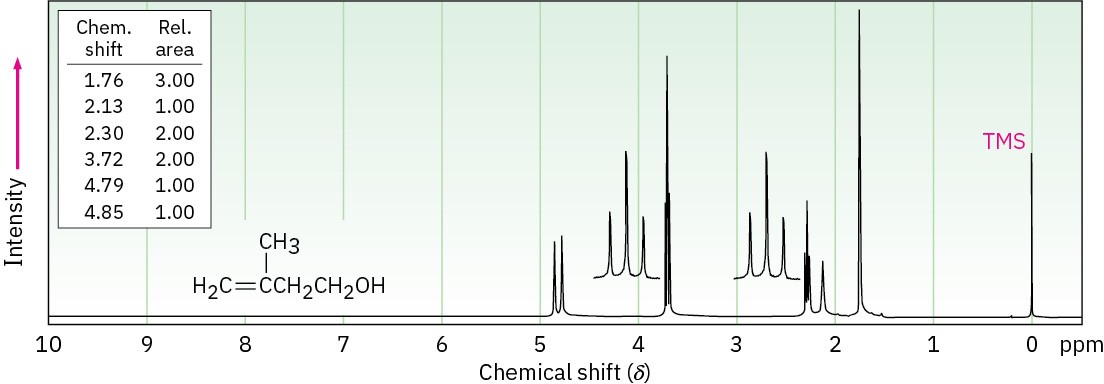
Problem 17-55
A compound of unknown structure gave the following spectroscopic data:
- Mass spectrum: M+ = 88.1
- IR: 3600 cm–1
- 1H NMR: 1.4 δ (2 H, quartet, J = 7 Hz); 1.2 δ (6 H, singlet); 1.0 δ (1 H, singlet); 0.9 δ (3 H, triplet, J = 7 Hz)
- 13C NMR: 74, 35, 27, 25 δ
(a)
Assuming that the compound contains C and H but may or may not contain O, give three possible molecular formulas.
(b)
How many hydrogens does the compound contain? (c)
What functional group(s) does the compound contain? (d)
How many carbons does the compound contain? (e)
What is the molecular formula of the compound? (f)
What is the structure of the compound? (g)
Assign peaks in the molecule’s 1H NMR spectrum corresponding to specific protons. Problem 17-56
Propose a structure for a compound C15H24O that has the following 1H NMR spectrum. The peak marked by an asterisk disappears when D2O is added to the sample.
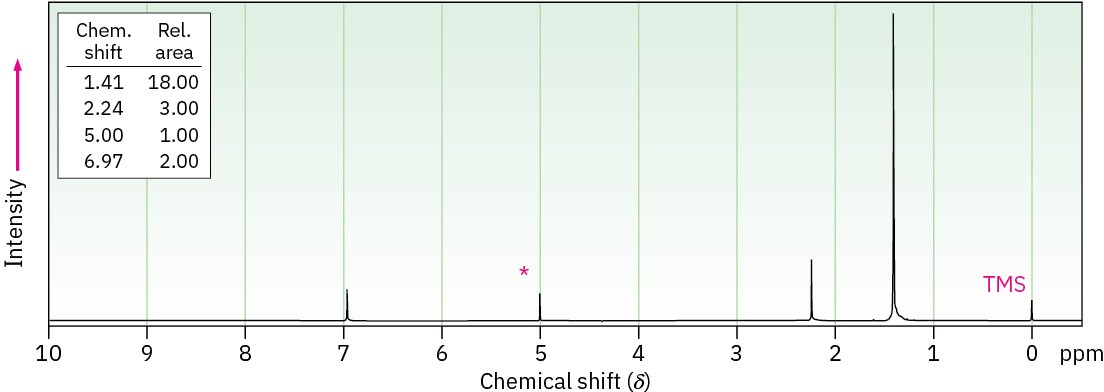
General Problems
Problem 17-57
How would you carry out the following transformations? (a)

(b)
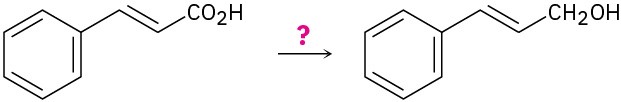
(c)

Problem 17-58
Benzoquinone is an excellent dienophile in the Diels–Alder reaction. What product would you expect from reaction of benzoquinone with 1 equivalent of 1,3-butadiene? From reaction with 2 equivalents of 1,3-butadiene?
Problem 17-59
Rank the following substituted phenols in order of increasing acidity, and explain your answer:
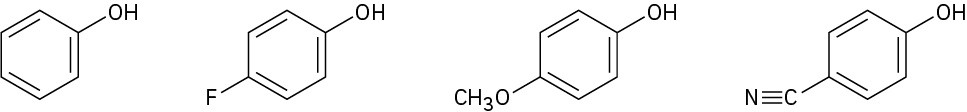
Problem 17-60
Benzyl chloride can be converted into benzaldehyde by treatment with nitromethane and base. The reaction involves initial conversion of nitromethane into its anion, followed by SN2 reaction of the anion with benzyl chloride and subsequent E2 reaction. Write the mechanism in detail, using curved arrows to indicate the electron flow in each step.
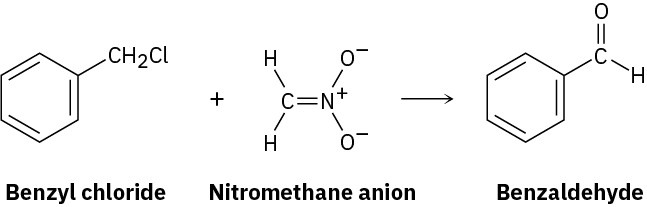
Problem 17-61
Reaction of (S)-3-methyl-2-pentanone with methylmagnesium bromide followed by acidification yields 2,3-dimethyl-2-pentanol. What is the stereochemistry of the product? Is the product optically active?

Problem 17-62
Testosterone is one of the most important male steroid hormones. When testosterone is dehydrated by treatment with acid, rearrangement occurs to yield the product shown. Propose a mechanism to account for this reaction.
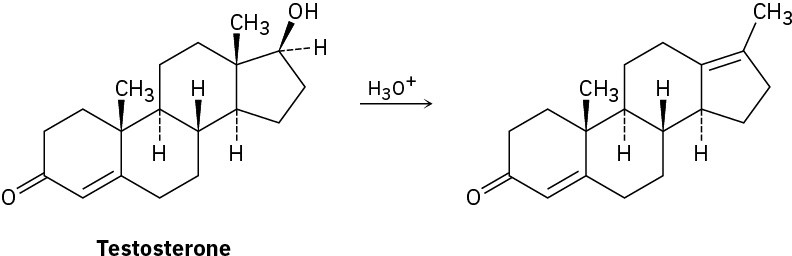
Problem 17-63
Starting from testosterone (Problem 17-62), how would you prepare the following substances?
(a)
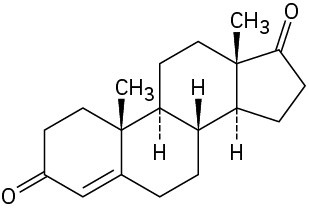
(b)
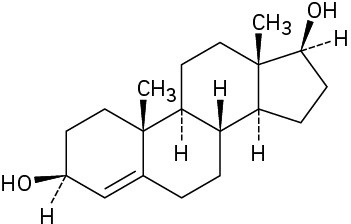
(c)
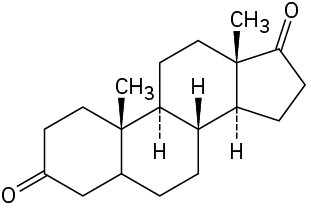
(d)
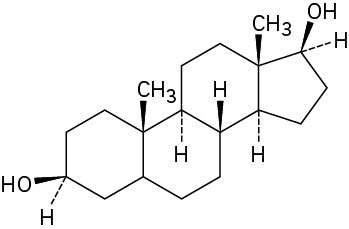
Problem 17-64
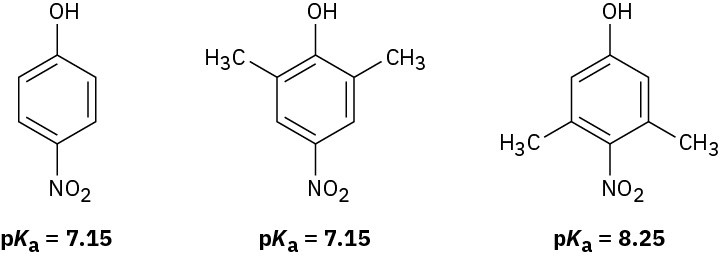
- Nitrophenol and 2,6-dimethyl-4-nitrophenol both have pKa = 7.15, but 3,5-dimethyl-4- nitrophenol has pKa = 8.25. Why is 3,5-dimethyl-4-nitrophenol so much less acidic?
Problem 17-65
Compound A, C10H18O, undergoes reaction with dilute H2SO4 at 25 °C to yield a mixture of two alkenes, C10H16. The major alkene product, B, gives only cyclopentanone after ozone
treatment followed by reduction with zinc in acetic acid. Write the reactions involved, and identify A and B.
Problem 17-66
Compound A, C5H10O, is one of the basic building blocks of nature. All steroids and many other naturally occurring compounds are built from compound A. Spectroscopic analysis of A yields the following information:
- IR: 3400 cm–1; 1640 cm–1
- 1H NMR: 1.63 δ (3 H, singlet); 1.70 δ (3 H, singlet); 3.83 δ (1 H, broad singlet); 4.15 δ
(2 H, doublet, J = 7 Hz); 5.70 δ (1 H, triplet, J = 7 Hz)
(a)
How many double bonds and/or rings does A have? (b)
From the IR spectrum, what is the identity of the oxygen-containing functional group? (c)
What kinds of hydrogens are responsible for the NMR absorptions listed? (d)
Propose a structure for A. Problem 17-67
Dehydration of trans-2-methylcyclopentanol with POCl3 in pyridine yields predominantly 3-methylcyclopentene. Is the stereochemistry of this dehydration syn or anti?
Problem 17-68
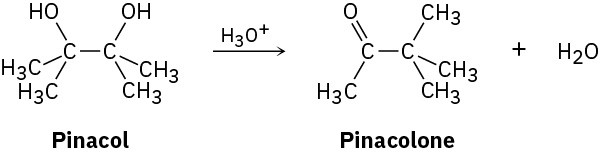
2,3-Dimethyl-2,3-butanediol has the common name pinacol. On heating with aqueous acid, pinacol rearranges to pinacolone, 3,3-dimethyl-2-butanone. Suggest a mechanism for this reaction.
Problem 17-69
As a rule, axial alcohols oxidize somewhat faster than equatorial alcohols. Which would you expect to oxidize faster, cis-4-tert-butylcyclohexanol or trans-4-tert-butylcyclohexanol?
Draw the more stable chair conformation of each molecule. Problem 17-70
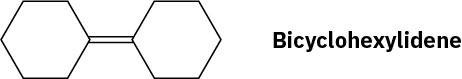
Propose a synthesis of bicyclohexylidene, starting from cyclohexanone as the only source of carbon.
Problem 17-71
A problem often encountered in the oxidation of primary alcohols to carboxylic acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate:
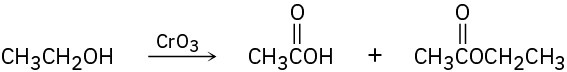
Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:
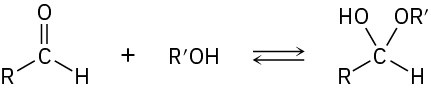
Problem 17-72
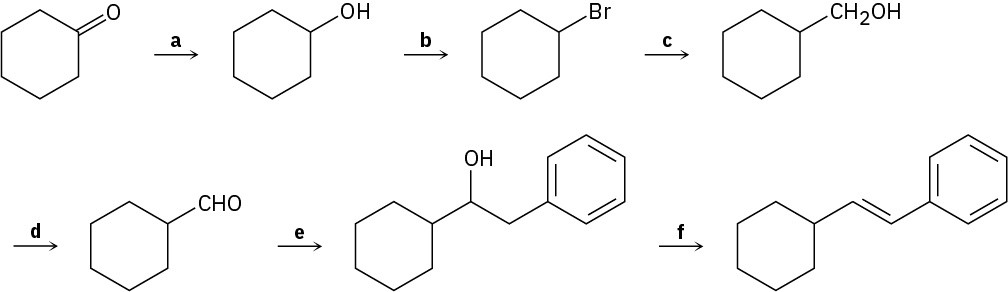
Identify the reagents a–f in the following scheme:
Problem 17-73
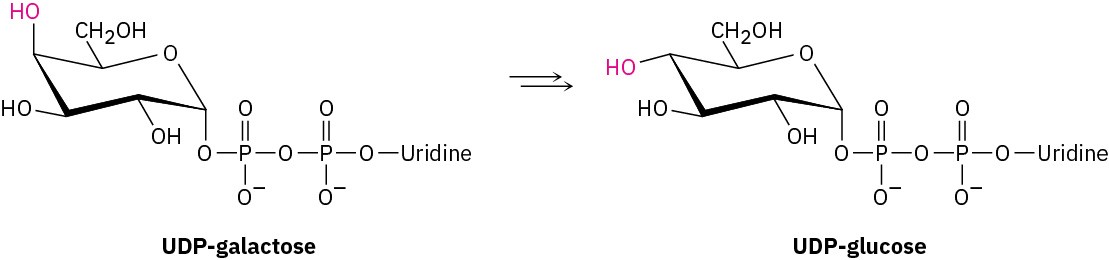
Galactose, a constituent of the disaccharide lactose found in dairy products, is metabolized by a pathway that includes the isomerization of UDP-galactose to UDP-glucose, where UDP
= uridylyl diphosphate. The enzyme responsible for the transformation uses NAD+ as cofactor. Propose a mechanism.
Problem 17-74
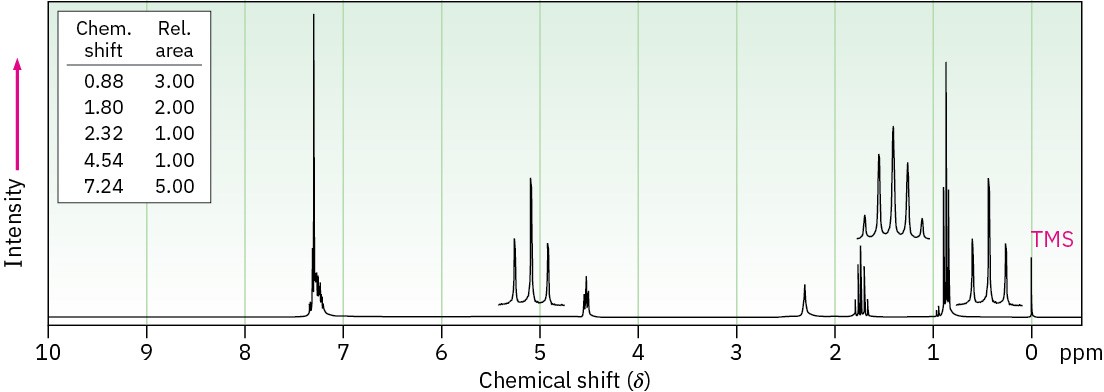
Propose structures for alcohols that have the following 1H NMR spectra: (a)
C9H12O
(b) C8H10O2
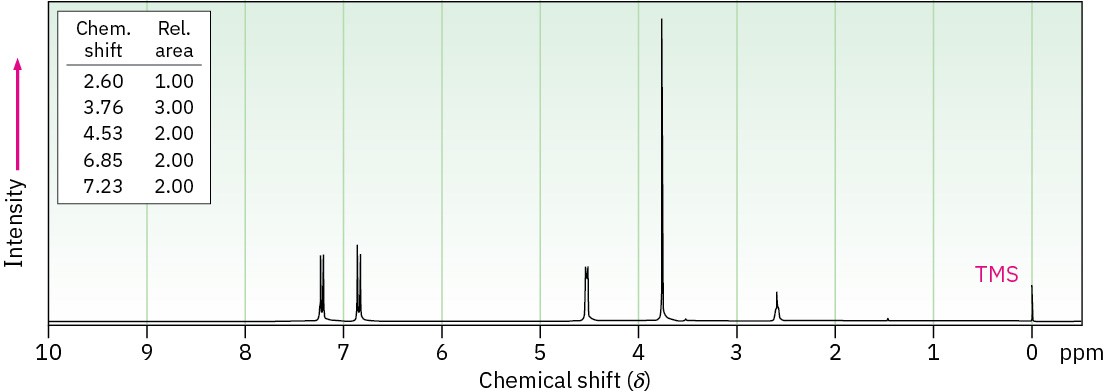
Problem 17-75
Compound A, C8H10O, has the IR and 1H NMR spectra shown. Propose a structure consistent with the observed spectra, and label each peak in the NMR spectrum. Note that the absorption at 5.5 δ disappears when D2O is added.

