10.4 Stability of the Allyl Radical: Resonance Revisited
To see why an allylic radical is so stable, look at the orbital picture in Figure 10.4. The radical carbon atom with an unpaired electron can adopt sp2 hybridization, placing the unpaired electron in a p orbital and giving a structure that is electronically symmetrical. The p orbital on the central carbon can therefore overlap equally well with a p orbital on either of the two neighboring carbons.
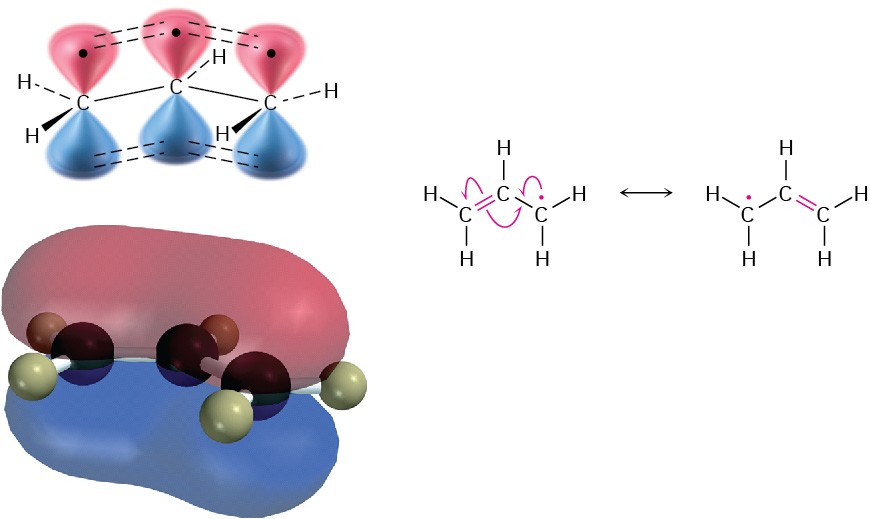
Figure 10.4 An orbital view of the allyl radical. The p orbital on the central carbon can overlap equally well with a p orbital on either neighboring carbon, giving rise to two equivalent resonance structures.
Because the allyl radical is electronically symmetrical, it has two resonance forms—one with the unpaired electron on the left and the double bond on the right and another with the unpaired electron on the right and the double bond on the left. Neither structure is correct by itself; the true structure of the allyl radical is a resonance hybrid of the two. (You might want to review Section 2.4 to Section 2.6 to brush up on resonance.) As noted in Section 2.5, the greater the number of resonance forms, the greater the stability of a compound, because bonding electrons are attracted to more nuclei. An allyl radical, with two resonance forms, is therefore more stable than a typical alkyl radical, which has only a single structure.
In molecular orbital terms, the stability of the allyl radical is due to the fact that the unpaired electron is delocalized, or spread out, over an extended π-orbital network rather than localized at only one site, as shown by the computer-generated MO in Figure 10.4.
This delocalization is particularly apparent in the so-called spin-density surface in Figure 10.5, which shows the calculated location of the unpaired electron. The two terminal carbons share the unpaired electron equally.
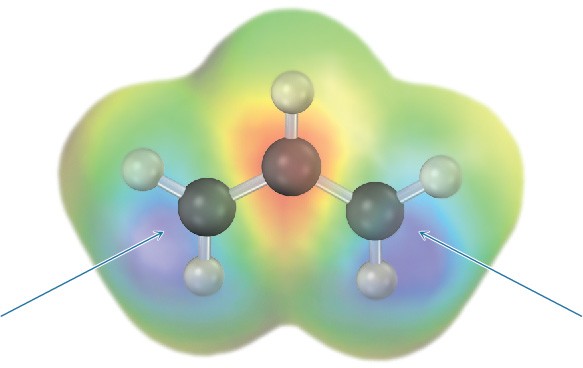
Figure 10.5The spin density surface of the allyl radical locates the position of the unpaired electron and shows that it is equally shared between the two terminal carbons.
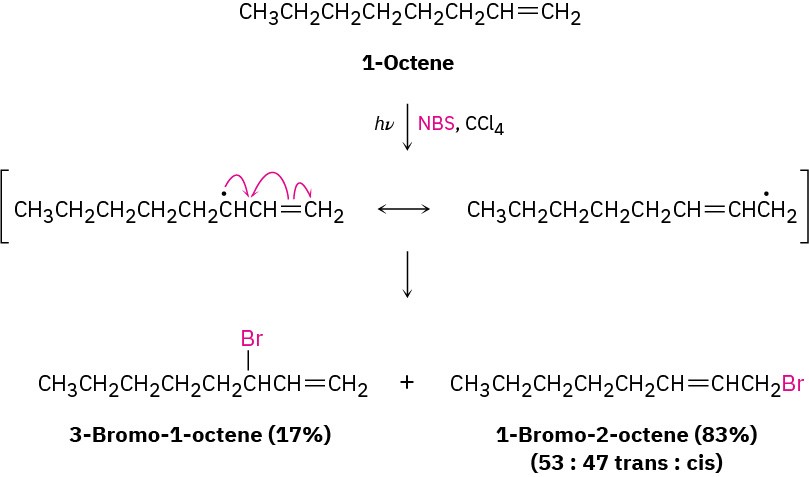
In addition to its effect on stability, delocalization of the unpaired electron in the allyl radical has other chemical consequences. Because the unpaired electron is delocalized over both ends of the π orbital system, reaction with Br2 can occur at either end. As a result, allylic bromination of an unsymmetrical alkene often leads to a mixture of products. For example, bromination of 1-octene gives a mixture of 3-bromo-1-octene and 1-bromo-2- octene. The two products are not formed in equal amounts, however, because the intermediate allylic radical is not symmetrical and reaction at the two ends is not equally likely. Reaction at the less hindered, primary end is favored.
The products of allylic bromination reactions are useful for conversion into dienes by dehydrohalogenation with base. Cyclohexene can be converted into 1,3-cyclohexadiene, for example.
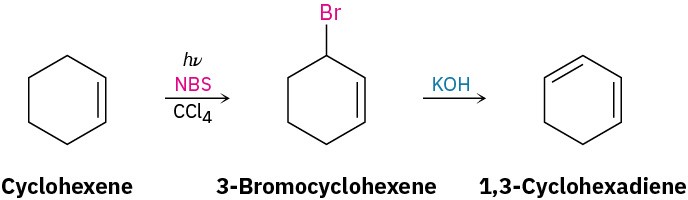
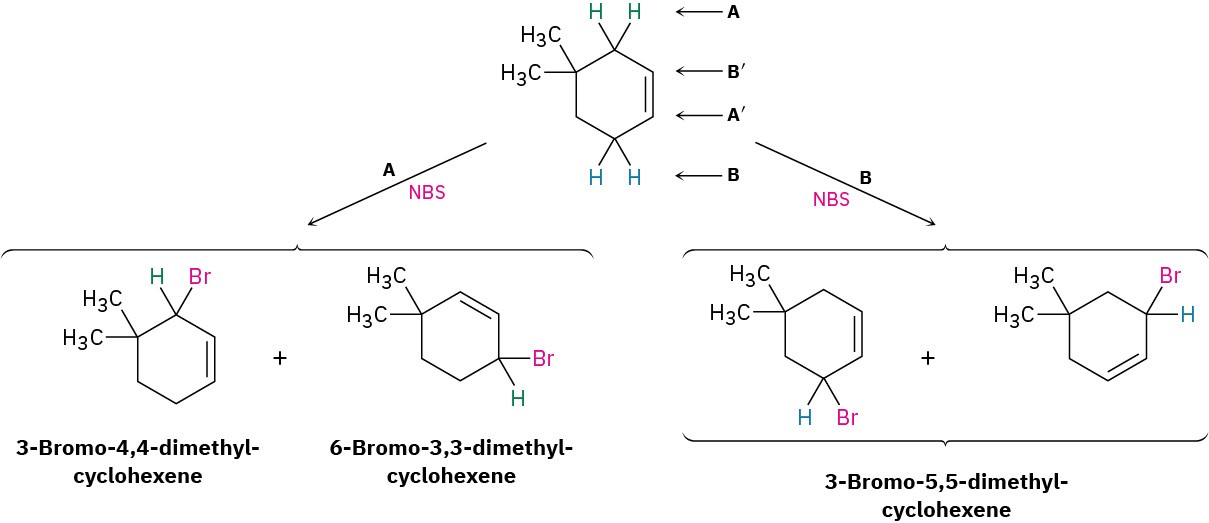
Worked Example 10.1Predicting the Product of an Allylic Bromination ReactionWhat products would you expect from the reaction of 4,4-dimethylcyclohexene with NBS?StrategyDraw the alkene reactant, and identify the allylic positions. In this case, there are two different allylic positions; we’ll label them A and B. Now abstract an allylic hydrogen from each position to generate the two corresponding allylic radicals. Each of the two allylic radicals can add a Br atom at either end (A or A′; B or B′), to give a mixture of up to four products. Draw and name the products. In the present instance, the “two” products from reaction at position B are identical, so only three products are formed in this reaction.Solution
Problem 10-5
Draw three resonance forms for the cyclohexadienyl radical.

Problem 10-6
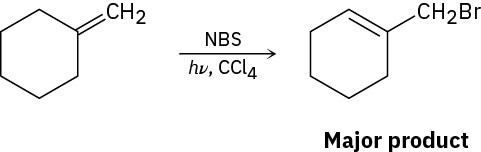
The major product of the reaction of methylenecyclohexane with N-bromosuccinimide is 1- (bromomethyl)cyclohexene. Explain.
Problem 10-7
What products would you expect from reaction of the following alkenes with NBS? If more than one product is formed, show the structures of all.
(a)
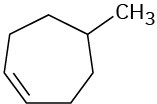
(b)