24 Why This Chapter?

Figure 24.1 The characteristic and unmistakable odor of fish is due to a mixture of simple alkylamines. (credit: modification of work “Pike Place” by Sharat Ganapati/Flickr, CC BY 2.0)
By the end of this chapter, we will have seen all the common functional groups. Of those groups, carbonyl compounds and amines are the most abundant and have the richest chemistry. In addition to proteins and nucleic acids, the majority of pharmaceutical agents
contain amine functional groups, and many of the common coenzymes necessary for biological catalysis are amines.
Amines are organic derivatives of ammonia in the same way that alcohols and ethers are organic derivatives of water. Like ammonia, amines contain a nitrogen atom with a lone pair of electrons, making amines both basic and nucleophilic. We’ll soon see, in fact, that most of the chemistry of amines depends on the presence of this lone pair of electrons.
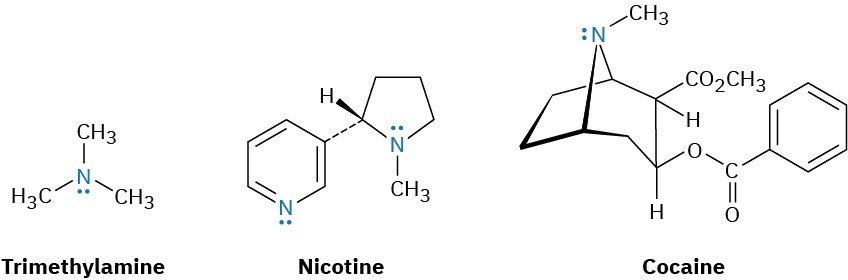
Amines occur widely in all living organisms. Trimethylamine, for instance, occurs in animal tissues and is partially responsible for the distinctive odor of fish; nicotine is found in tobacco; and cocaine is a stimulant found in the leaves of the South American coca bush. In addition, amino acids are the building blocks from which all proteins are made, and cyclic amine bases are constituents of nucleic acids.