4 Why This Chapter?
Figure 4.1 The musk gland of the male Himalayan musk deer secretes a substance once used in perfumery that contains cycloalkanes of 14 to 18 carbons. (credit: modification of work “Siberian musk deer in the tiaga” by ErikAdamsson/Wikimedia Commons, CC0 1.0)
We’ll see numerous instances in future chapters where the chemistry of a given functional group is affected by being in a ring rather than an open chain. Because cyclic molecules are encountered in most pharmaceuticals and in all classes of biomolecules, including proteins, lipids, carbohydrates, and nucleic acids, it’s important to understand the behavior of cyclic structures.
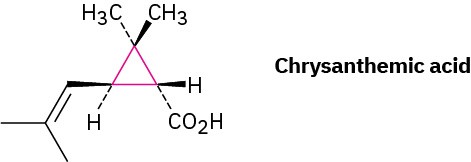
Although we’ve only discussed open-chain compounds up to now, most organic compounds contain rings of carbon atoms. Chrysanthemic acid, for instance, whose esters occur naturally as the active insecticidal constituents of chrysanthemum flowers, contains a three-membered (cyclopropane) ring.
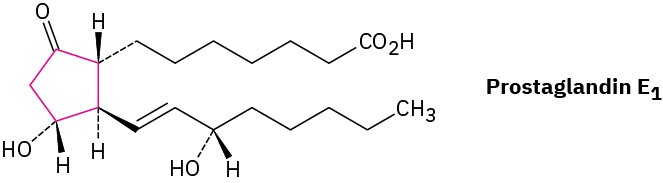
Prostaglandins, potent hormones that control an extraordinary variety of physiological functions in humans, contain a five-membered (cyclopentane) ring.
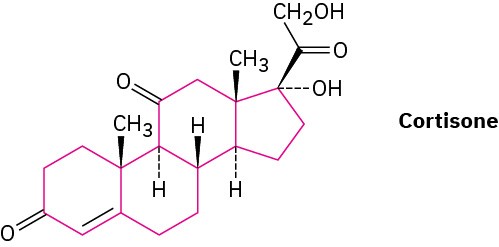
Steroids, such as cortisone, contain four rings joined together—three six-membered (cyclohexane) and one five-membered. We’ll discuss steroids and their properties in more detail in Sections 27.6 and 27.7.