5 Why This Chapter?

Figure 5.1 Like the mountain whose image is reflected in a lake, many organic molecules also have mirror-image counterparts. (credit: modification of work “Crystal Lake sunrise reflection” by Sandy Horvath-Dori/Wikimedia Commons, CC BY 2.0)
Understanding the causes and consequences of molecular handedness is crucial to understanding organic and biological chemistry. The subject can be a bit complex at first, but the material covered in this chapter nevertheless forms the basis for much of the remainder of the book.
Are you right-handed or left-handed? You may not spend much time thinking about it, but handedness plays a surprisingly large role in your daily activities. Many musical instruments, such as oboes and clarinets, have a handedness to them; the last available softball glove always fits the wrong hand. The reason for these difficulties is that our hands aren’t identical; rather, they’re mirror images. When you hold a left hand up to a mirror, the image you see looks like a right hand. Try it.
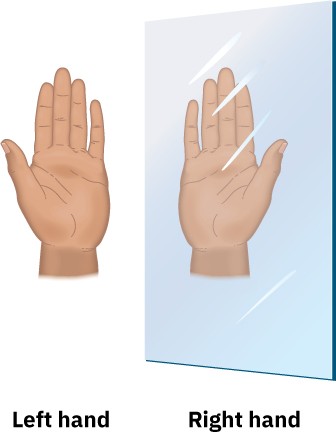
Handedness is also important in organic and biological chemistry, where it arises primarily as a consequence of the tetrahedral stereochemistry of sp3-hybridized carbon atoms. Many drugs and almost all the molecules in our bodies—amino acids, carbohydrates, nucleic acids, and many more—have a handedness. Furthermore, molecular handedness enables the precise interactions between enzymes and their substrates that are involved in the hundreds of thousands of chemical reactions on which life is based.

